Modulation of human endogenous retrovirus (HERV) transcription during persistent and de novo HIV-1 infection
- PMID: 25886562
- PMCID: PMC4375885
- DOI: 10.1186/s12977-015-0156-6
Modulation of human endogenous retrovirus (HERV) transcription during persistent and de novo HIV-1 infection
Abstract
Background: The human genome contains multiple LTR elements including human endogenous retroviruses (HERVs) that together account for approximately 8-9% of the genomic DNA. At least 40 different HERV groups have been assigned to three major HERV classes on the basis of their homologies to exogenous retroviruses. Although most HERVs are silenced by a variety of genetic and epigenetic mechanisms, they may be reactivated by environmental stimuli such as exogenous viruses and thus may contribute to pathogenic conditions. The objective of this study was to perform an in-depth analysis of the influence of HIV-1 infection on HERV activity in different cell types.
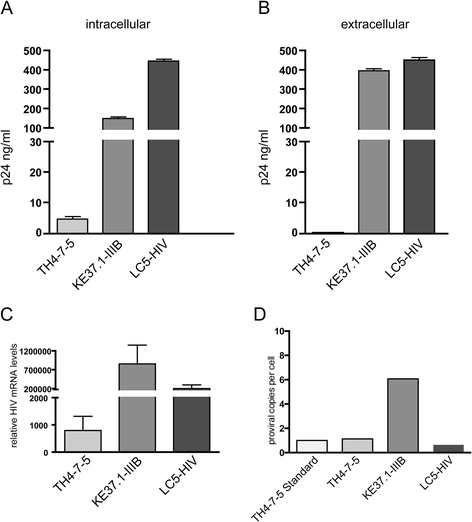
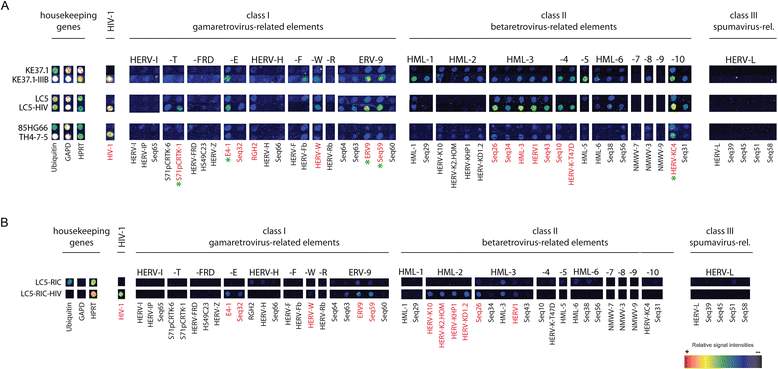
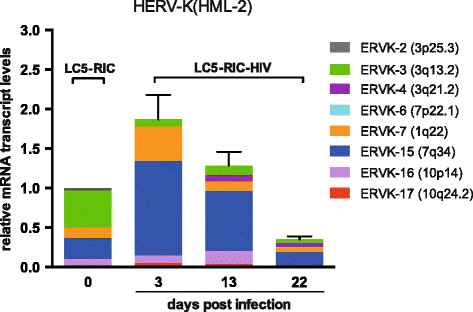
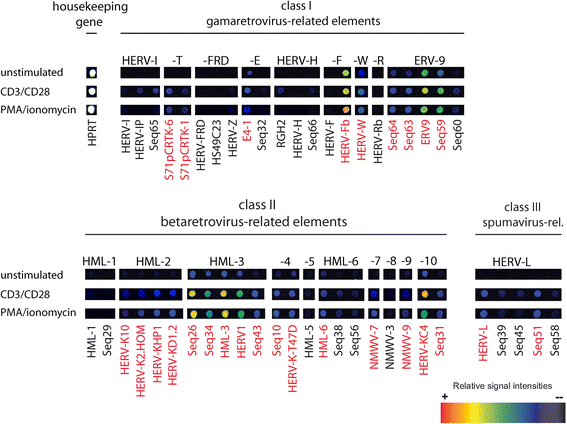
Results: A retrovirus-specific microarray that covers major HERV groups from all three classes was used to analyze HERV transcription patterns in three persistently HIV-1 infected cell lines of different cellular origins and in their uninfected counterparts. All three persistently infected cell lines showed increased transcription of multiple class I and II HERV groups. Up-regulated transcription of five HERV taxa (HERV-E, HERV-T, HERV-K (HML-10) and two ERV9 subgroups) was confirmed by quantitative reverse transcriptase PCR analysis and could be reversed by knock-down of HIV-1 expression with HIV-1-specific siRNAs. Cells infected de novo by HIV-1 showed stronger transcriptional up-regulation of the HERV-K (HML-2) group than persistently infected cells of the same origin. Analysis of transcripts from individual members of this group revealed up-regulation of predominantly two proviral loci (ERVK-7 and ERVK-15) on chromosomes 1q22 and 7q34 in persistently infected KE37.1 cells, as well as in de novo HIV-1 infected LC5 cells, while only one single HML-2 locus (ERV-K6) on chromosome 7p22.1 was activated in persistently infected LC5 cells.
Conclusions: Our results demonstrate that HIV-1 can alter HERV transcription patterns of infected cells and indicate a correlation between activation of HERV elements and the level of HIV-1 production. Moreover, our results suggest that the effects of HIV-1 on HERV activity may be far more extensive and complex than anticipated from initial studies with clinical material.
Figures



References
-
- Sverdlov E. Retroviruses and primate genome evolution. Georgetown, Texas, USA: Landes Bioscience; 2005.
-
- Mager DL, Medstrand P. Retroviral repeat sequences. In: Cooper D, editor. Nature encyclopedia of the human genome. D. Cooper edition. London, United Kingdom: Nature Publishing Group; 2003. pp. 57–63.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

