A preliminary randomized double blind placebo-controlled trial of intravenous immunoglobulin for Japanese encephalitis in Nepal
- PMID: 25886645
- PMCID: PMC4401695
- DOI: 10.1371/journal.pone.0122608
A preliminary randomized double blind placebo-controlled trial of intravenous immunoglobulin for Japanese encephalitis in Nepal
Erratum in
-
Correction: A Preliminary Randomized Double Blind Placebo-Controlled Trial of Intravenous Immunoglobulin for Japanese Encephalitis in Nepal.PLoS One. 2015 Aug 13;10(8):e0136008. doi: 10.1371/journal.pone.0136008. eCollection 2015. PLoS One. 2015. PMID: 26270465 Free PMC article. No abstract available.
Abstract
Background: Japanese encephalitis (JE) virus (JEV) is a mosquito-borne flavivirus found across Asia that is closely related to West Nile virus. There is no known antiviral treatment for any flavivirus. Results from in vitro studies and animal models suggest intravenous immunoglobulin (IVIG) containing virus-specific neutralizing antibody may be effective in improving outcome in viral encephalitis. IVIG's anti-inflammatory properties may also be beneficial.
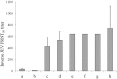
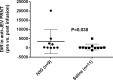
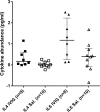
Methodology/principal findings: We performed a pilot feasibility randomized double-blind placebo-controlled trial of IVIG containing anti-JEV neutralizing antibody (ImmunoRel, 400mg/kg/day for 5 days) in children with suspected JE at two sites in Nepal; we also examined the effect on serum neutralizing antibody titre and cytokine profiles. 22 children were recruited, 13 of whom had confirmed JE; 11 received IVIG and 11 placebo, with no protocol violations. One child (IVIG group) died during treatment and two (placebo) subsequently following hospital discharge. Overall, there was no difference in outcome between treatment groups at discharge or follow up. Passive transfer of anti-JEV antibody was seen in JEV negative children. JEV positive children treated with IVIG had JEV-specific neutralizing antibody titres approximately 16 times higher than those treated with placebo (p=0.2), which was more than could be explained by passive transfer alone. IL-4 and IL-6 were higher in the IVIG group.
Conclusions/significance: A trial of IVIG for JE in Nepal is feasible. IVIG may augment the development of neutralizing antibodies in JEV positive patients. IVIG appears an appealing option for JE treatment that warrants further study.
Trial registration: ClinicalTrials.gov NCT01856205.
Conflict of interest statement
Figures
References
-
- Solomon T, Dung NM, Kneen R, Thao le TT, Gainsborough M, Nisalak A, et al. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain: a journal of neurology. 2002;125(Pt 5):1084–93. Epub 2002/04/19. PubMed . - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

