Statistical power and validity of Ebola vaccine trials in Sierra Leone: a simulation study of trial design and analysis
- PMID: 25886798
- PMCID: PMC4815262
- DOI: 10.1016/S1473-3099(15)70139-8
Statistical power and validity of Ebola vaccine trials in Sierra Leone: a simulation study of trial design and analysis
Abstract
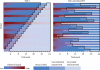
Background: Safe and effective vaccines could help to end the ongoing Ebola virus disease epidemic in parts of west Africa, and mitigate future outbreaks of the virus. We assess the statistical validity and power of randomised controlled trial (RCT) and stepped-wedge cluster trial (SWCT) designs in Sierra Leone, where the incidence of Ebola virus disease is spatiotemporally heterogeneous, and is decreasing rapidly.
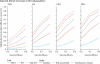
Methods: We projected district-level Ebola virus disease incidence for the next 6 months, using a stochastic model fitted to data from Sierra Leone. We then simulated RCT and SWCT designs in trial populations comprising geographically distinct clusters at high risk, taking into account realistic logistical constraints, and both individual-level and cluster-level variations in risk. We assessed false-positive rates and power for parametric and non-parametric analyses of simulated trial data, across a range of vaccine efficacies and trial start dates.
Findings: For an SWCT, regional variation in Ebola virus disease incidence trends produced increased false-positive rates (up to 0·15 at α=0·05) under standard statistical models, but not when analysed by a permutation test, whereas analyses of RCTs remained statistically valid under all models. With the assumption of a 6-month trial starting on Feb 18, 2015, we estimate the power to detect a 90% effective vaccine to be between 49% and 89% for an RCT, and between 6% and 26% for an SWCT, depending on the Ebola virus disease incidence within the trial population. We estimate that a 1-month delay in trial initiation will reduce the power of the RCT by 20% and that of the SWCT by 49%.
Interpretation: Spatiotemporal variation in infection risk undermines the statistical power of the SWCT. This variation also undercuts the SWCT's expected ethical advantages over the RCT, because an RCT, but not an SWCT, can prioritise vaccination of high-risk clusters.
Funding: US National Institutes of Health, US National Science Foundation, and Canadian Institutes of Health Research.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




Comment in
-
Ebola vaccine trials: a race against the clock.Lancet Infect Dis. 2015 Jun;15(6):624-6. doi: 10.1016/S1473-3099(15)70159-3. Epub 2015 Apr 14. Lancet Infect Dis. 2015. PMID: 25886800 No abstract available.
References
-
- Mohammadi D. Ebola vaccine trials back on track. Lancet. 2015;385:214–5. - PubMed
-
- Centers for Disease Control and Prevention . ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2015. STRIVE (Sierra Leone Trial to Introduce a Vaccine Against Ebola). Available from https://clinicaltrials.gov/ct2/show/NCT02378753 NLM Identifier: NCT02378753.
-
- Enserink M. High hopes for Guinean vaccine trial. Science (80-) 2015;347:219–20. - PubMed
-
- Van der Graaf R, van Delden JJM. Equipoise should be amended, not abandoned. Clin Trials. 2011;8:408–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

