Propofol, midazolam, vancomycin and cyclosporine therapeutic drug monitoring in extracorporeal membrane oxygenation circuits primed with whole human blood
- PMID: 25886890
- PMCID: PMC4335544
- DOI: 10.1186/s13054-015-0772-5
Propofol, midazolam, vancomycin and cyclosporine therapeutic drug monitoring in extracorporeal membrane oxygenation circuits primed with whole human blood
Abstract
Introduction: As a result of drug sequestration and increased volume of distribution, the extracorporeal membrane oxygenation (ECMO) procedure might lead to a decrease in drug concentrations during a patient's treatment. The aim of this study was to evaluate sedative, antibiotic and immunosuppressive drug loss in ECMO circuit using ex-vivo and in-vitro experiments.
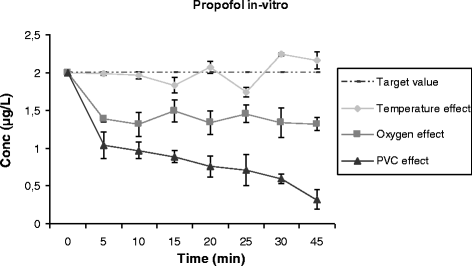
Methods: Blood concentrations of propofol, midazolam, cyclosporine and vancomycin were measured in an ex-vivo ECMO circuit primed with whole human blood, and compared to controls stored in polypropylene tubes. In vitro experiments were also conducted to further explore the role of temperature, oxygen exposure and polyvinylchloride surfaces on propofol loss in the ECMO circuit.
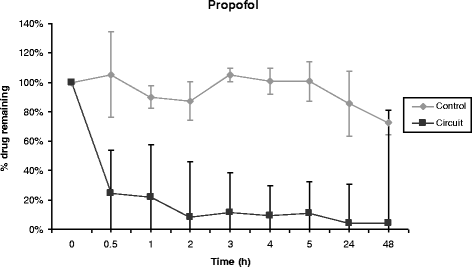
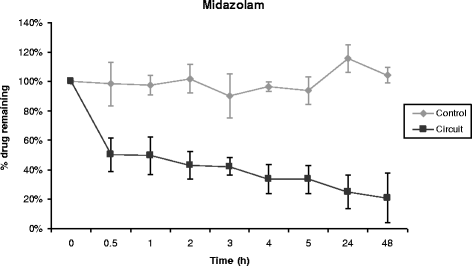
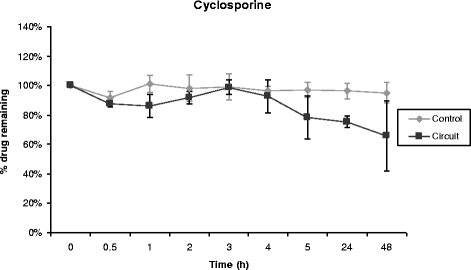
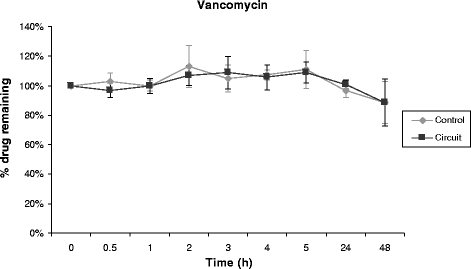
Results: Propofol concentration decreased rapidly; 70% of its baseline concentration was lost after only 30 minutes, and only 11% remained after five hours (P <0.001 for the comparison with control polypropylene tube propofol concentration). Further experiments demonstrated that oxygen exposure and contact with polyvinylchloride tubing were respectively responsible for 70% and 85% of propofol loss after 45 minutes. Midazolam concentration also rapidly decreased in the ECMO circuit, with only 54% and 11% of baseline concentration being detected at 30 minutes and 24 hours respectively (P = 0.01 versus control). Alternatively, cyclosporine concentration remained stable for the five first hours, then decreased to 78% and 73% of the baseline value after 24 hours and 48 hours, (P = 0.35 versus control). Lastly, vancomycin concentration remained stable in the ECMO circuit for the 48-hour experimental protocol.
Conclusions: We observed important losses of propofol and midazolam, while cyclosporine concentration decreased slowly and moderately, and vancomycin concentration remained unchanged in the ex-vivo ECMO circuit primed with whole human blood. These data might help intensive care unit physicians planning clinical trials with a final objective to better adapt doses of these drugs while treating critically ill ECMO patients.
Figures
References
-
- Shekar K, Fraser JF, Smith MT, Roberts JA. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care. 2012;27(741):e9–18. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

