Improved Tacrolimus Target Concentration Achievement Using Computerized Dosing in Renal Transplant Recipients--A Prospective, Randomized Study
- PMID: 25886918
- PMCID: PMC4591080
- DOI: 10.1097/TP.0000000000000708
Improved Tacrolimus Target Concentration Achievement Using Computerized Dosing in Renal Transplant Recipients--A Prospective, Randomized Study
Abstract
Background: Early after renal transplantation, it is often challenging to achieve and maintain tacrolimus concentrations within the target range. Computerized dose individualization using population pharmacokinetic models may be helpful. The objective of this study was to prospectively evaluate the target concentration achievement of tacrolimus using computerized dosing compared with conventional dosing performed by experienced transplant physicians.
Methods: A single-center, prospective study was conducted. Renal transplant recipients were randomized to receive either computerized or conventional tacrolimus dosing during the first 8 weeks after transplantation. The median proportion of tacrolimus trough concentrations within the target range was compared between the groups. Standard risk (target, 3-7 μg/L) and high-risk (8-12 μg/L) recipients were analyzed separately.
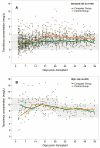
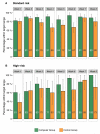
Results: Eighty renal transplant recipients were randomized, and 78 were included in the analysis (computerized dosing (n = 39): 32 standard risk/7 high-risk, conventional dosing (n = 39): 35 standard risk/4 high-risk). A total of 1711 tacrolimus whole blood concentrations were evaluated. The proportion of concentrations per patient within the target range was significantly higher with computerized dosing than with conventional dosing, both in standard risk patients (medians, 90% [95% confidence interval {95% CI}, 84-95%] vs 78% [95% CI, 76-82%], respectively, P < 0.001) and in high-risk patients (medians, 77% [95% CI, 71-80%] vs 59% [95% CI, 40-74%], respectively, P = 0.04).
Conclusions: Computerized dose individualization improves target concentration achievement of tacrolimus after renal transplantation. The computer software is applicable as a clinical dosing tool to optimize tacrolimus exposure and may potentially improve long-term outcome.
Trial registration: ClinicalTrials.gov NCT02010320.
Figures
References
-
- Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transplant. 2014;14(Suppl 1):11. - PubMed
-
- Wallemacq P, Armstrong VW, Brunet M, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: Report of the European consensus conference. Ther Drug Monit. 2009;31:139. - PubMed
-
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623. - PubMed
-
- Saint-Marcoux F, Woillard J-B, Jurado C, Marquet P. Lessons from routine dose adjustment of tacrolimus in renal transplant patients based on global exposure. Ther Drug Monit. 2013;35:322. - PubMed
-
- Staatz C, Taylor P, Tett S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol Dial Transplant. 2001;16:1905. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

