The Effect of Cinacalcet on Calcific Uremic Arteriolopathy Events in Patients Receiving Hemodialysis: The EVOLVE Trial
- PMID: 25887067
- PMCID: PMC4422249
- DOI: 10.2215/CJN.10221014
The Effect of Cinacalcet on Calcific Uremic Arteriolopathy Events in Patients Receiving Hemodialysis: The EVOLVE Trial
Abstract
Background and objectives: Uncontrolled secondary hyperparathyroidism (sHPT) in patients with ESRD is a risk factor for calcific uremic arteriolopathy (CUA; calciphylaxis).
Design, setting, participants, & measurements: Adverse event reports collected during the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events trial were used to determine the frequency of CUA in patients receiving hemodialysis who had moderate to severe sHPT, as well as the effects of cinacalcet versus placebo. CUA events were collected while patients were receiving the study drug.
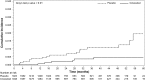
Results: Among the 3861 trial patients who received at least one dose of the study drug, 18 patients randomly assigned to placebo and six assigned to cinacalcet developed CUA (unadjusted relative hazard, 0.31; 95% confidence interval [95% CI], 0.13 to 0.79; P=0.014). Corresponding cumulative event rates (95% CI) at year 4 were 0.011% (0.006% to 0.018%) and 0.005% (0.002% to 0.010%). By multivariable analysis, other factors associated with CUA included female sex, higher body mass index, higher diastolic BP, and history of dyslipidemia or parathyroidectomy. Median (10%, 90% percentile) plasma parathyroid hormone concentrations proximal to the report of CUA were 796 (225, 2093) pg/ml and 410 (71, 4957) pg/ml in patients randomly assigned to placebo and cinacalcet, respectively. Active use of vitamin K antagonists was recorded in 11 of 24 patients with CUA, nine randomly assigned to placebo, and two to cinacalcet, in contrast to 5%-7% at any one time point in patients in whom CUA was not reported.
Conclusion: Cinacalcet appeared to reduce the incidence of CUA in hemodialysis recipients who have moderate to severe sHPT.
Trial registration: ClinicalTrials.gov NCT00345839.
Keywords: calcium receptor; dialysis; hyperparathyroidism; hyperphosphatemia; mineral metabolism.
Copyright © 2015 by the American Society of Nephrology.
Figures
Comment in
-
Evolving calciphylaxis--what randomized, controlled trials can contribute to the capture of rare diseases.Clin J Am Soc Nephrol. 2015 May 7;10(5):726-8. doi: 10.2215/CJN.03350315. Epub 2015 Apr 17. Clin J Am Soc Nephrol. 2015. PMID: 25887071 Free PMC article. No abstract available.
References
-
- Brandenburg VM, Sinha S, Specht P, Ketteler M: Calcific uraemic arteriolopathy: A rare disease with a potentially high impact on chronic kidney disease-mineral and bone disorder. Pediatr Nephrol 29: 2289–2298, 2014 - PubMed
-
- Coates T, Kirkland GS, Dymock RB, Murphy BF, Brealey JK, Mathew TH, Disney AP: Cutaneous necrosis from calcific uremic arteriolopathy. Am J Kidney Dis 32: 384–391, 1998 - PubMed
-
- Fine A, Zacharias J: Calciphylaxis is usually non-ulcerating: Risk factors, outcome and therapy. Kidney Int 61: 2210–2217, 2002 - PubMed
-
- Mazhar AR, Johnson RJ, Gillen D, Stivelman JC, Ryan MJ, Davis CL, Stehman-Breen CO: Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int 60: 324–332, 2001 - PubMed
-
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR: Calciphylaxis: Natural history, risk factor analysis, and outcome. J Am Acad Dermatol 56: 569–579, 2007 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical