TNF-alpha inhibitors for ankylosing spondylitis
- PMID: 25887212
- PMCID: PMC11200207
- DOI: 10.1002/14651858.CD005468.pub2
TNF-alpha inhibitors for ankylosing spondylitis
Abstract
Background: TNF (tumor necrosis factor)-alpha inhibitors block a key protein in the inflammatory chain reaction responsible for joint inflammation, pain, and damage in ankylosing spondylitis.
Objectives: To assess the benefit and harms of adalimumab, etanercept, golimumab, and infliximab (TNF-alpha inhibitors) in people with ankylosing spondylitis.
Search methods: We searched the following databases to January 26, 2009: MEDLINE (from 1966); EMBASE (from 1980); the Cochrane Central Register of Controlled Trials (CENTRAL; 2008, Issue 4); ACP Journal Club; CINAHL (from 1982); and ISI Web of Knowledge (from 1900). We ran updated searches in May 2012, October 2013, and in June 2014 for McMaster PLUS. We searched major regulatory agencies for safety warnings and clinicaltrials.gov for registered trials.
Selection criteria: Randomized controlled trials (RCTs) comparing adalimumab, etanercept, golimumab and infliximab to placebo, other drugs or usual care in patients with ankylosing spondylitis, reported in abstract or full-text.
Data collection and analysis: Two authors independently assessed search results, risk of bias, and extracted data. We conducted Bayesian mixed treatment comparison (MTC) meta-analyses using WinBUGS software. To investigate a class-effect of harms across biologics, we pooled harms data using Review Manager 5.
Main results: We included twenty-one, short-term (24 weeks or less) RCTs with a total of 3308 participants; 18 contributed data to the MTC analysis: adalimumab (4 studies), etanercept (8 studies), golimumab (2 studies), infliximab (3 studies), and one head-to-head study (etanercept versus infliximab) which was unblinded and considered at a higher risk of bias. The risk of selection and detection bias was low or unclear for most of the studies. The risk of selective outcome reporting was low for most studies as they reported on outcomes recommended by the Assessment of SpondyloArthritis international Society. We found little heterogeneity and no significant inconsistency in the MTC analyses. The majority of the studies were funded by pharmaceutical companies. Most studies permitted concomitant therapy of stable doses of disease-modifying anti-rheumatic drugs, non-steroidal anti-inflammatory drugs, or corticosteroids, but allowances varied across studies.Compared with placebo, there was high quality evidence that patients on an anti-TNF agent were three to four times more likely to achieve an ASAS40 response (assessing spinal pain, function, and inflammation, as measured by the mean of intensity and duration of morning stiffness, and patient global assessment) by six months (adalimumab: risk ratio (RR) 3.53, 95% credible interval (Crl) 2.49 to 4.91; etanercept: RR 3.31, 95% Crl 2.38 to 4.53; golimumab: RR 2.90, 95% Crl 1.90 to 4.23; infliximab: RR 4.07, 95% Crl 2.80 to 5.74, with a 25% to 40% absolute difference between treatment and placebo groups. The number needed to treat (NNT) to achieve an ASAS 40 response ranged from 3 to 5.There was high quality evidence of improvement in physical function on a 0 to 10 scale (adalimumab: mean difference (MD) -1.6, 95% Crl -2.2 to -0.9; etanercept: MD -1.1, 95% CrI -1.6 to -0.6; golimumab: MD -1.5, 95% Crl -2.3 to -0.7; infliximab: MD -2.1, 95% Crl -2.7 to -1.4, with an 11% to 21% absolute difference between treatment and placebo groups. The NNT to achieve the minimally clinically important difference of 0.7 points ranged from 2 to 4.Compared with placebo, there was moderate quality evidence (downgraded for imprecision) that patients on an anti-TNF agent were more likely to achieve an ASAS partial remission by six months (adalimumab: RR 6.28, 95% Crl 3.13 to 12.78; etanercept: RR 4.24, 95% Crl 2.31 to 8.09; golimumab: RR 5.18, 95% Crl 1.90 to 14.79; infliximab: RR 15.41, 95% Crl 5.09 to 47.98 with a 10% to 44% absolute difference between treatment and placebo groups. The NNT to achieve an ASAS partial remission response ranged from 3 to 11.There was low to moderate level evidence of a greater reduction in spinal inflammation as measured by magnetic resonance imaging though the absolute differences were small and the clinical relevance of the difference was unclear: adalimumab (1 trial; -6% (95% confidence interval (CI) -12% to 0.05%); 1 trial: 53.6% mean decrease from baseline versus 9.4% mean increase in the placebo group), golimumab (1 trial; -2.5%, (95% CI -5.6% to -0.7%)), and infliximab (1 trial; -3% (95% CI -4% to -2.4%)).Radiographic progression was measured in one trial (N = 60) of etanercept versus placebo and it found that radiologic changes were similar in both groups (detailed data not provided).There were few events of withdrawals due to adverse events leading to imprecision around the estimates. When all the anti-TNF agents were combined against placebo, there was moderate quality evidence from 16 studies of an increased risk of withdrawals due to adverse events in the anti-TNF group (Peto odds ratio (OR) 2.44, 95% CI 1.26 to 4.72; total events: 38/1637 in biologic group; 7/986 in placebo) though the absolute increase in harm was small (1%; 95% CI 0% to 2%).Due to low event rates, evidence of the effect of individual TNF-inhibitors against placebo or for all four biologics pooled together versus placebo on serious adverse events is inconclusive (moderate quality; downgraded for imprecision). For all anti-TNF pooled versus placebo based on 16 studies: Peto OR 1.45, 95% CI 0.85 to 2.48; 51/1530 in biologic group; 18/878 in placebo; absolute difference: 1% (95% CI 0% to 2%).Using indirect comparison methodology, and one head-to-head study of etanercept versus infliximab, wide confidence intervals meant that results were inconclusive for evidence of differences in the major outcomes between different anti-TNF agents. Regulatory agencies have published warnings about rare adverse events of serious infections, including tuberculosis, malignancies and lymphoma.
Authors' conclusions: There is moderate to high quality evidence that anti-TNF agents improve clinical symptoms in the treatment of ankylosing spondylitis. More participants withdrew due to adverse events when on an anti-TNF agent but we did not find evidence of an increase in serious adverse events, though event rates were low and trials had a short duration. The short-term toxicity profile appears acceptable. Based on indirect comparison methodology, we are uncertain whether there are differences between anti-TNF agents in terms of the key benefit or harm outcomes.
Conflict of interest statement
We received a National Institute for Health Research (NIHR) Cochrane Incentive Award to assist with the completion of this review.
LJM: was an associate member of the Assessment of SpondyloArthritis international Society from 2005 to 2014.
JZ: none known
AB: Grants: to department only: Abbvie, Pfizer, Merck, Amgen Travel support to department: Janssen‐Cilag; Honorarium: part to department, part personally: Pfizer, UCB, Janssen‐Cilag, Abbvie
JAS: received research grants from Takeda and Savient and consultant fees from Savient, Takeda, Regeneron and Allergan. JAS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms‐length funding from 36 companies; a member of the American College of Rheumatology's Guidelines Subcommittee of the Quality of Care Committee; and a member of the Veterans Affairs Rheumatology Field Advisory Committee.
ETG: none known.
MV: none known.
MBJ: none known.
PT: grants/honoraria from Bristol Myers, Chiltern International, and UCB.
GW: none known
Figures
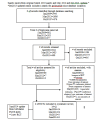


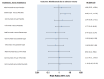
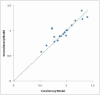



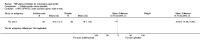








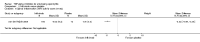




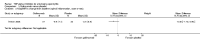
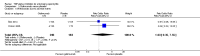
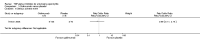












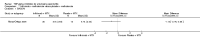


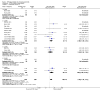

Update of
- doi: 10.1002/14651858.CD005468
References
References to studies included in this review
Bao 2014 {published data only}
-
- Bao C, Huang F, Khan MA, Fei K, Zhong W, Han C, et al. Safety and efficacy of golimumab in Chinese patients with active ankylosing spondylitis: 1‐year results of a multicentre, randomized, double‐blind, placebo‐controlled phase III trial. Rheumatology 2014;53:1654‐63. - PubMed
Barkham 2010 {published data only}
-
- Barkham N, Coates L, Keen H, Hensor E, Fraser A, Redmond A, et al. A double blind placebo controlled trial of etanercept in the prevention of work disability in ankylosing spondylitis. Rheumatology 2008;47:ii72. - PubMed
-
- Barkham N, Coates LC, Keen H, Hensor E, Fraser A, Redmond A, et al. Double‐blind placebo‐controlled trial of etanercept in the prevention of work disability in ankylosing spondylitis. Annals of the Rheumatic Diseases 2010;69(11):1926‐8. - PubMed
Brandt 2003 {published and unpublished data}
-
- Brandt J, Khariouzov A, Listing J, Haibel H, Sorensen H, Grassnickel L. Six‐month results of a double‐blind, placebo‐controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis and Rheumatism 2003;48:1667‐75. - PubMed
Braun 2002 {published and unpublished data}
-
- Braun J, Brandt J, Listing J, Zink A, Alten R, Krause A, et al. Treatment of active ankylosing spondylitis with infliximab, a double‐blind placebo controlled multicenter trial. Lancet 2002;359:1187‐93. - PubMed
Braun 2011 {published data only}
-
- Braun J, Burgos‐Vargas R, Horst‐Bruinsma IE, Freundlich B, Vlahos B, Koenig AS, et al. Assessment of clinical efficacy in a randomized, double‐blind study of etanercept and sulphasalazine in patients with ankylosing spondylitis. Arthritis and Rheumatism. 2008; Vol. 58:S415. - PubMed
-
- Braun J, Pavelka K, Ramos‐Remus C, Dimic A, Vlahos B, Freundlich B, et al. Clinical efficacy of etanercept versus sulfasalazine in ankylosing spondylitis subjects with peripheral joint involvement. Journal of Rheumatology 2012;39(4):836‐40. - PubMed
-
- Braun J, Horst‐Bruinsma IE, Huang F, Burgos‐Vargas R, Vlahos B, Koenig AS, et al. Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: A randomized, double‐blind trial. Arthritis and Rheumatism 2011;63(6):1543‐51. - PubMed
-
- Sieper J, Moots R, Taylor A, Sato R, Singh A, Roberson D, et al. Assessment of patient‐reported outcomes from a randomized, double‐blind study of etanercept and sulphasalazine in patients with ankylosing spondylitis. Arthritis and Rheumatism. 2008; Vol. 58:S580.
Calin 2004 {published and unpublished data}
Davis 2003 {published data only (unpublished sought but not used)}
-
- Davis J, Heijde D, Braun J, Dougados M, Cush J, Clegg D, et al. Recombinant Human Tumor Necrosis Factor Receptor (Etanercept) for Treating Ankylosing Spondylitis. Arthritis and Rheumatism 2003;48(11):3230‐6. - PubMed
-
- Davis J, Heijde, Braun J, Dougados M, Clegg DO, Kivits A, et al. Effiacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis. Annals of the Rheumatic Diseases. 2008; Vol. 67:346‐52. - PubMed
Dougados 2011 {published data only}
-
- Dougados M, Braun J, Szanto S, Combe B, Elbaz M, Geher P, et al. Efficacy of etanercept on rheumatic signs and pulmonary function tests in advanced ankylosing spondylitis: Results of a randomised double‐blind placebo‐controlled study (SPINE). Annals of the Rheumatic Diseases 2011;70:799‐804. [DOI: 10.1136/ard.2010.139261] - DOI - PMC - PubMed
Giardina 2009 {published data only}
-
- Braun J, Baraliakos X, Hermann KG, Heijde D, Inman RD, Deodhar AA, et al. Golimumab reduces spinal inflammation in ankylosing spondylitis: MRI results of the randomised, placebo‐controlled GO‐RAISE study. Annals of the Rheumatic Diseases 2012;71(6):878‐84. [DOI: 10.1136/annrheumdis‐2011‐200308] - PMC - PubMed
-
- Giardina AR, Ferrante A, Ciccia F, Impastato R, Miceli M, Principato A, et al. A 2‐year comparative open label randomized study of efficacy and safety of etanercept and infliximab in patients with ankylosing spondylitis. Rheumatology International 2009 October 23 [Epub ahead of print]. [DOI: 10.1007/s00296-009-1157-3] - DOI - PubMed
-
- Impastato R, Ciccia F, Ferrante A, Principato A, Giardina A, Cadelo M, et al. Infliximab and etanercept are both effective and safe in patients with ankylosing spondylitis. A two‐year randomised study. Annals of the Rheumatic Diseases 2007;66(Suppl II):399.
Gorman 2002 {published data only}
-
- Gorman JD, Sack KE, Davis JC. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. New England Journal of Medicine 2002;346:1349‐56. - PubMed
Hu 2012 {published data only}
-
- Hu Z, Xu M, Qiuxia L, Zhiming L, Liao Z, Cao S, et al. Adalimumab significantly reduces inflammation and serumDKK‐1 level but increases fatty deposition in lumbar spine inactive ankylosing spondylitis. International Journal of Rheumatic Diseases 2012;15:358‐65. - PubMed
Huang 2008 {published data only}
-
- Huang F, MacPeek D, Shunle C, Li Z, Gu J, Wu D, et al. Etanercept is effective and well tolerated in Chinese patients with ankylosing spondylitis: 6‐week placebo‐controlled trial followed by 6‐week open‐label treatment. Annals of the Rheumatic Diseases. 2008; Vol. 67:Suppl II: 521.
-
- Huang F, Zhang J, Huang JL, Wu DH, Li ZG, Chen SL, et al. A multicenter, double‐blind, placebo‐controlled, randomized, phase III clinical study of etanercept in treatment of ankylosing spondylitis. Zhonghua Nei Ke Za Zhi (Chinese Journal of Internal Medicine) 2010;49:741–5. - PubMed
Huang 2014 {published data only}
Inman 2008 {published data only}
-
- Braun J, Baraliakos X, Hermann KG, Heijde D, Inman RD, Deodhar AA, et al. Golimumab reduces spinal inflammation in ankylosing spondylitis: MRI results of the randomised, placebo‐controlled GO‐RAISE study. Annals of the Rheumatic Diseases 2012;71(6):878‐84. [DOI: 10.1136/annrheumdis-2011-200308] - DOI - PMC - PubMed
-
- Inman RD, Davis JC Jr, Heijde Dv, Diekman L, Sieper J, Kim SI, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double‐blind, placebo‐controlled, phase III trial. Arthritis and Rheumatism 2008;58(11):3402‐12. - PubMed
Inman 2010 {published data only}
-
- Inman R, Maksymowych W, CANDLE group. A double‐blind, placebo‐controlled trial of low dose infliximab in ankylosing spondylitis. Journal of Rheumatology 2010;37(6):1‐8. [DOI: ] - PubMed
-
- Inman R, Shojania K, Keystone E, Haraoui B, Mitchell C, Maksymowych W. CANaDian evaluation of Low dosE infliximab in ankylosing spondylitis (CANDLE). Journal of Rheumatology 2008;35(6):1213‐14.
-
- Maksymowych W, Salonen D, Inman R, Paul FL, Lambert RG. CANaDian evaluation of Low dosE Infliximab in Ankylosing Spondylitis (CANDLE) ‐ 12 week magnetic resonance imaging evaluation of spinal inflammation with the SPARCC MRI method. Journal of Rheumatology 2008;35(6):1211‐12.
-
- Maksymowych WP, Salonen D, Inman RD, Rahman P, Lambert RGW. Low‐dose infliximab (3 mg/kg) significantly reduces spinal inflammation on magnetic resonance imaging in patients with ankylosing spondylitis: a randomized placebo‐controlled study. Journal of Rheumatology 2010;37(8):1728‐34. - PubMed
Lambert 2007 {published data only}
-
- Lambert RGW, Salonen D, Rahman P, Inman RD, Wong RL, Einstein SG, et al. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis. Arthritis and Rheumatism 2007;56(12):4005‐14. - PubMed
-
- Maksymowych W, Rahman P, Keystone E, Wong R, Inman R, M03‐606 Study Group. Efficacy of adalimumab in active AS: Results from the Canadian AS study. Arthritis and Rheumatism. 2005:S217.
-
- Maksymowych W, Rahman P, Shojania K, Olszynski W, Thomson G, Ballal S, et al. Beneficial effects of adalimumab on biomarkers reflecting structural damage in patients with ankylosing spondylitis. Journal of Rheumatology 2008;35(10):2030‐7. - PubMed
Marzo‐Ortega 2005 {published data only}
Navarro‐Sarabia 2011 {published data only}
-
- Navarro‐Sarabia F, Fernandez‐Sueiro JL, Torre‐Alonso JC, Gratacos J, Queiro R, Gonzalez C, et al. High‐dose etanercept in ankylosing spondylitis: results of a 12‐week randomized, double blind, controlled multicentre study (LOADET study). Rheumatology 2011;50(10):1828‐37. - PubMed
van der Heijde 2005 {published data only}
-
- Braun J, Deodhar A, Dijkmans B, Geusens P, Sieper J, Williamson P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis over a two‐year period. Arthritis and Rheumatism 2008;59:1270‐8. - PubMed
-
- Braun J, Landewe R, Hermann KGA, Han J, Yan S, Williamson P, et al. Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab ‐ Results of a multicenter, randomized, double‐blind, placebo‐controlled magnetic resonance imaging study. Arthritis and Rheumatism 2006;54(5):1646‐52. - PubMed
-
- Heldmann F, Brandt J, Braun J, EASIC Study Group. European ankylosing spondylitis infliximab cohort (EASIC): safety of longterm therapy with infliximab in patients with ankylosing spondylitis. Annals of the Rheumatic Diseases. 2008; Vol. 67:Suppl II:386.
-
- Heldmann F, Brandt J, Horst‐Bruinsma, Landewe R, Sieper J, Burmester G, et al. European Ankylosing Spondylitis Infliximab Cohort (EASIC): Safety of longterm therapy in patients with ankylosing spondylitis. Annals of the Rheumatic Diseases. 2009; Vol. 68 (Suppl 3):626.
-
- Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, et al. Efficacy and Safety of Infliximab in Patients with Ankylosing Spondylitis. Results of a Randomized, Placebo‐Controlled Trial (ASSERT). Arthritis and Rheumatism 2005;52(2):582‐91. - PubMed
van der Heijde 2006a {published and unpublished data}
-
- Davis Jr JC, Revicki D, Heijde DMF, Rentz AM, Wong RL, Kupper H, et al. Health‐related quality of life outcomes in patients with active ankylosing spondylitis treated with adalimumab: Results from a randomized controlled study. Arthritis Care and Research 2007; Vol. 57, issue 6:1050‐7. [DOI: 10.1002/art.22887] - DOI - PubMed
-
- Revicki DA, Luo MP, Wordsworth P, Wong RL, Chen N, Davis Jr J. Adalimumab reduces pain, fatigue, and stiffness in patients with ankylosing spondylitis: Results from the adalimumab trial evaluating long‐term safety and efficacy for ankylosing spondylitis (ATLAS). Journal of Rheumatology 2008;35(7):1346‐53. - PubMed
-
- Heijde D, Dijkmans B, Schiff M, Kivitz A, Vlam K, Wordsworth P, et al. Maintenance of reduction in disease activity and partial remission in patients with ankylosing spondylitis (AS) ‐ 3 year results from the adalimumab (Humira) trial evaluating long‐term efficacy and safety in AS (ATLAS). Arthritis and Rheumatism. 2008:S579.
-
- Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BAC, Braun J, ATLAS study group. Efficacy and safety of adalimumab in patients with ankylosing spondylitis. Arthritis and Rheumatism 2006;54(7):2136‐46. - PubMed
van der Heijde 2006b {published data only}
-
- Braun J, McHugh N, Singh A, Wajdula JS, Sato R. Improvement in patient‐reported outcomes for patients with ankylosing spondylitis treated with etanercept 50 mg once‐weekly and 25 mg twice‐weekly. Rheumatology 2007;46(6):999‐1004. - PubMed
References to studies excluded from this review
Barkham 2008b {published data only}
-
- Barkham N, Keen HI, Coates P, O'Connor EMA, Hensor AD, Fraser LS, et al. A randomized controlled trial of infliximab shows clinical and MRI efficacy in HLA B27 positive very early ankylosing spondylitis. Annals of the Rheumatic Diseases. 2008; Vol. 67:Suppl II:382.
Breban 2008 {published data only}
-
- Breban M, Ravaud P, Claudepierre P, Baron G, Henry Y‐D, Hudry C, et al. Maintenance of infliximab treatment in ankylosing spondylitis: Results of a one‐year randomized controlled trial comparing systematic versus on‐demand treatment. Arthritis and Rheumatism 2008;58(1):88‐97. [DOI: 10.1002/art.23167] - DOI - PubMed
Haibel 2008 {published data only}
-
- Haibel H, Rudwaleit M, Listing J, Heldmann F, Wong RL, Kupper H, et al. Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: Results of a twelve‐week randomized, double‐blind, placebo‐controlled trial followed by an open‐label extension up to week fifty‐two. Arthritis and Rheumatism 2008; Vol. 58, issue 7:1981‐91. [DOI: 10.1002/art.23606] - DOI - PubMed
Li 2008 {published data only}
-
- Li EK, Griffth JF, Lee VW, Wang YX, Li TK, Lee KK, et al. Short‐term effiacy of combination methotrexate and infliximab in patients with ankylosing spondylitis: a clinical and magnetic resonance imaging correlation. Rheumatology 2008;47(9):1358‐63. - PubMed
Morency 2011 {published data only}
-
- Morency N, Lambert R, Maksymowych W. Direct imaging evidence that adalimumab induces resolution of inflammatory lesions in AS patients at sites of complete spinal ankylosis. Journal of Rheumatology 2011;38(6):1138.
Van den Bosch 2002 {published data only}
-
- Bosch F, Kruithof E, Baeten D, Herssens A, Keyser F, Mielants H, et al. Randomized double‐blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondyloarthropathy. Arthritis and Rheumatism 2002;46(3):755‐65. - PubMed
References to studies awaiting assessment
Zhang 2009 {published data only}
-
- Zhang J, Zhang YM, Zhang J‐L, Deng X‐H, Huang F. Efficacy of etanercept in patients with ankylosing spondylitis: A double‐blind, randomized, placebo controlled trial. [Chinese]. Chinese Journal of New Drugs 2009;18(19):1846.
Additional references
Ades 2008
-
- Ades AE, Welton NJ, Caldwell D, Price M, Goubar A, Lu G. Multiparameter evidence synthesis in epidemiology and medical decision‐making. Journal of Health Services Research Policy 2008;13 Suppl 3:12‐22. - PubMed
Anderson 2001
-
- Anderson JJ, Baron G, Heijde D, Felson DT, Dougados M. Ankylosing Spondylitis Assessment group preliminary definition of short‐term improvement in ankylosing spondylitis. Arthritis and Rheumatism 2001;44(8):1876‐86. - PubMed
Boers 2006
-
- Boers M. Abatacept in rheumatoid arthritis: a new branch on the biologics tree (editorial). Annals of the Rheumatic Diseases 2006;144:933‐5. - PubMed
Boonen 2001a
Boonen 2001b
Bradburn 2007
-
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26(1):53‐77. - PubMed
Brandt 2000
-
- Brandt J, Haibel H, Cornely D, Golder W, Gonzalez J, Reddig J, et al. Successful treatment of active ankylosing spondylitis with the anti‐tumor necrosis factor alpha monoclonal antibody infliximab. Arthritis and Rheumatism 2000;43(6):1346‐52. - PubMed
Brandt 2004
Braun 1995
-
- Braun J, Bollow M, Neure L, Seipelt E, Seyrekbasan F, Herbst H, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis and Rheumatism 1995;38(4):499‐505. - PubMed
Braun 2003
Braun 2006
-
- Braun J, Landewe R, Hermann KGA, Han J, Yan S, Williamson P, et al. Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab ‐ Results of a multicenter, randomized, double‐blind, placebo‐controlled magnetic resonance imaging study. Arthritis and Rheumatism 2006;54(5):1646‐52. - PubMed
Braun 2012a
-
- Braun J, Pavelka K, Ramos‐Remus C, Dimic A, Vlahos B, Freundlich B, et al. Clinical efficacy of etanercept versus sulfasalazine in ankylosing spondylitis subjects with peripheral joint involvement. Journal of Rheumatology 2012;39(4):836‐40. - PubMed
Braun 2012b
-
- Braun J, Baraliakos X, Hermann K‐G A, Heijde D, Inman RD, Deodhar AA, et al. Golimumab reduces spinal inflammation in ankylosing spondylitis: MRI results of the randomised, placebo‐ controlled GO‐RAISE study. Annals of the Rheumatic Diseases 2012;71:878‐84. [DOI: 10.1136/annrheumdis-2011-200308] - DOI - PMC - PubMed
Bucher 1997
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. - PubMed
Calin 1994
-
- Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. Journal of Rheumatology 1994;21(12):2281‐85. - PubMed
Callhoff 2014
Cates 2008 [Computer program]
-
- Dr. Chris Cates. Visual Rx3. Dr Chris Cates' EBM website www.nntonline.com. Dr. Chris Cates, 2008.
Chen 2014
Cooper 2009
-
- Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between‐study heterogeneity and inconsistency in mixed treatment comparisons: application to stroke prevention treatments in individuals with non‐rheumatic atrial fibrillation. Statistics in Medicine 2009;28(14):1861‐81. - PubMed
Dagfinrud 2008
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29:932‐44. [DOI: 10.1002/sim.3767] - PubMed
Dias 2011a
-
- Dias S, Welton NJ, Sutton AJ, Ades AE, National Institute for Health and Clinical Excellence, Decision Support Unit. NICE DSU technical support document 1: introduction to evidence synthesis for decision making. www.cc‐arcc.ca/common/pages/UserFile.aspx?fileId=282147 (accessed 22 Apr 2014).
Dias 2011b
-
- Dias S, Welton NJ, Sutton AJ, Ades AE, National Institute for Health and Clinical Excellence, Decision Support Unit. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta‐analysis of randomised controlled trials. www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%2015Apri... (accessed 22 Apr 2014). - PubMed
Dias 2011c
-
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE, National Institute for Health and Clinical Excellence, Decision Support Unit. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials. www.nicedsu.org.uk/TSD4%20Inconsistency.final.15April2014.pdf (accessed 22 Apr 2014). - PubMed
Dougados 2002
Edmunds 1991
-
- Edmunds L, Elswood J, Kennedy LG, Calin A. Primary ankylosing spondylitis, psoriatic and enteropathic spondyloarthropathy: a controlled analysis. Journal of Rheumatology 1991;18(5):696‐8. - PubMed
GRADEpro 2014 [Computer program]
-
- McMaster University. GRADEpro. [www.gradepro.org]. McMaster University, Version 3.6 [2014].
Haibel 2004
-
- Haibel H, Brandt HC, Rudwaleit M, Listing J, Braun J, Kupper H, et al. Efficacy and safety of adalimumab in the treatment of active ankylosing spondylitis: Preliminary results of an open‐label, 20‐week trial. Arthritis and Rheumatism 2004;50(Suppl):S217.
Haibel 2010
-
- Haibel H, Sieper J. Editorial review: how early should ankylosing spondylitis be treated with a tumor necrosis factor‐blocker?. Current Opinion in Rheumatology 2010;22:388‐92. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hochberg 2003
-
- Hochberg MC, Tracy JK, Hawkins‐Holt M, Flores RH. Comparison of the efficacy of the tumour necrosis factor (alpha) blocking agents adalimumab, etanercept, and infliximab when added to methotrexate in patients with active rheumatoid arthritis. Annals of the Rheumatic Diseases 2003;62(Suppl 2):ii13‐ii16. - PMC - PubMed
Inman 2011
-
- Inman RD. The spondyloarthropathies. In: Goldman L, Schafer AI editor(s). Cecil Medicine. 24th Edition. Philadelphia, PA: Elsevier Saunders, 2011.
Khan 2002
-
- Khan M. Update on spondyloarthropathies. Annals of Internal Medicine 2002;136:896‐907. - PubMed
Lord 2010
Maksymowych 2002
-
- Maksymowych W, Jhangri G, Lambert R, Mallon C, Buenviaje H, Pedryca E, et al. Infliximab in ankylosing spondylitis: a prospective observational inception cohort analysis of efficacy and safety. Journal of Rheumatology 2002;29:959‐65. - PubMed
Maksymowych 2003
-
- Maksymowych W, Inman R, Gladman D, Thompson G, Stone M, Karsh J, et al. Canadian Rheumatolgy Association Consensus on the use of anti‐tumor necrosis factor‐alpha directed therapies in the treatment of spondyloarthritis. Journal of Rheumatology 2003;30:1356‐63. - PubMed
Maksymowych 2005
-
- Maksymowych W, Rahman P, Keystone E, Wong R, Inman R, M03‐606 Study Group. Efficacy of adalimumab in active AS: Results from the Canadian AS study. Arthritis and Rheumatism. 2005:S217.
Maksymowych 2008
-
- Maksymowych W, Rahman P, Shojania K, Olszynski W, Thomson G, Ballal S, et al. Beneficial effects of adalimumab on biomarkers reflecting structural damage in patients with ankylosing spondylitis. Journal of Rheumatology 2008;35(10):2030‐7. - PubMed
Maksymowych 2010
-
- Maksymowych WP, Salonen D, Inman RD, Rahman P, Lambert RGW. Low‐dose infliximab (3 mg/kg) significantly reduces spinal inflammation on magnetic resonance imaging in patients with ankylosing spondylitis: a randomized placebo‐controlled study. Journal of Rheumatology 2010;37(8):1728‐34. - PubMed
Marzo‐Ortega 2001
-
- Marzo‐Ortega H, McGonagle D, O'Connor P, Emery P. Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondylarthropathy: a clinical and magnetic resonance imaging study. Arthritis and Rheumatism 2001;44(9):2112‐7. - PubMed
Montacer 2009
NICE 2008
-
- National Institute for Health and Clinical Excellence. Adalimumab, etanercept and infliximab for ankylosing spondylitis. NICE technology appraisal guidance 143. www.nice.org.uk/TA143 (accessed January 2010).
NICE 2011
-
- National Institute for Health and Clinical Excellence. Golimumab for the treatment of ankylosing spondylitis: NICE technology appraisal guidance 233. www.nice.org.uk/guidance/ta233/resources/guidance‐golimumab‐for‐the‐trea... 2011.
Pavy 2005
-
- Pavy S, Brophy S, Calin A. Establishment of the minimum clinically important difference for the bath ankylosing spondylitis indices: a prospective study. Journal of Rheumatology 2005;32(1):80‐5. - PubMed
Plot Digitizer 2014 [Computer program]
-
- Huwaldt JA, Steinhorst S. Plot Digitizer. Open source; http://plotdigitizer.sourceforge.net/, 27 April 2014.
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rudwaleit 2004
Rudwaleit 2009a
-
- Rudwaleit M, Landewe R, Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Annals of the Rheumatic Diseases 2009;68:770‐6. - PubMed
Rudwaleit 2009b
-
- Rudwaleit M, Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Annals of the Rheumatic Diseases 2009;68:777‐83. - PubMed
Schultz 1995
-
- Schultz K, Chalmers I, Hayes R, Altman D. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Schünemann 2009
-
- Schünemann H, Brożek J, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2. The GRADE Working Group. updated March 2009, issue Completing the Summary of Findings for an Outcome: Dichotomous outcomes ‐ Corresponding risk.
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Song 2009
Spiegelhalter 2003
-
- Spiegelhalter D, Thomas A, Best N, Lunn D. WinBUGS user manual [Internet]. Version 1.4. Cambridge, UK: MRC Biostatistics Unit, Institute of Public Health (accessed 21 Apr 2014).
Stone 2001
-
- Stone M, Salonen D, Lax M, Payne U, Lapp V, Inman R. Clinical and imaging correlates of response to treatment with infliximab in patients with ankylosing spondylitis. Journal of Rheumatology 2001;28:1605‐14. - PubMed
Sweeting 2004
-
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23:1351‐75. - PubMed
Turner 2005
-
- Turner RM, Thompson SG, Spiegelhalter DJ. Prior distributions for the intracluster correlation coefficient, based on multiple previous estimates, and their application in cluster randomized trials. Clinical Trials 2005;2(2):108‐18. - PubMed
Van Den Bosch 2002
-
- Bosch F, Kruithof E, Baeten D, Herssens A, Keyser F, Mielants H, et al. Randomized double‐blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis and Rheumatism 2002;46:755‐65. - PubMed
van der Heijde 1997
-
- Heijde D, Bellamy N, Calin A, Dougados M, Khan MA, Linden S. Preliminary core sets for endpoints in ankylosing spondylitis. Journal of Rheumatology 1997;24:2225‐9. - PubMed
van der Heijde 2011
-
- Heijde D, Sieper J, Maksymowych WP, Dougados M, Burgos‐Vargas R, Landewé R, et al. Assessment of SpondyloArthritis international Society. 2010 Update of the international ASAS recommendations for the use of anti‐TNF agents in patients with axial spondyloarthritis. Annals of the Rheumatic Diseases 2011;70(6):905‐8. [DOI: 10.1136/ard.2011.151563] - DOI - PubMed
Wanders 2005
-
- Wanders A, Heijde D, Landewe R, Behier JM, Calin A, Olivieri I, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis. Arthritis and Rheumatism 2005;52(6):1756‐65. - PubMed
Wells 2009
-
- Wells GA, Sultan SA, Chen L, Khan M, Coyle D, Canadian Agency for Drugs and Technologies in Health. Indirect Evidence: Indirect Treatment Comparisons in Meta‐Analysis. www.cadth.ca/media/pdf/H0462_itc_tr_e.pdf 2009.
Yazici 2008
-
- Yazici Y, Adler NM, Yazici H. Most tumour necrosis factor inhibitor trials in rheumatology are undeservedly called 'efficacy and safety' trials: a survey of power considerations. Rheumatology 2008;47(7):1054‐7. - PubMed
Zink 2000
-
- Zink A, Braun J, Listing J, Wollenhaupt J. Disability and handicap in rheumatoid arthritis and ankylosing spondylitis ‐ results from the German rheumatological database. Journal of Rheumatology 2000;27:613‐32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

