Spatial, temporal and genetic dynamics of highly pathogenic avian influenza A (H5N1) virus in China
- PMID: 25887370
- PMCID: PMC4329208
- DOI: 10.1186/s12879-015-0770-x
Spatial, temporal and genetic dynamics of highly pathogenic avian influenza A (H5N1) virus in China
Abstract
Background: The spatial spread of H5N1 avian influenza, significant ongoing mutations, and long-term persistence of the virus in some geographic regions has had an enormous impact on the poultry industry and presents a serious threat to human health.
Methods: We applied phylogenetic analysis, geospatial techniques, and time series models to investigate the spatiotemporal pattern of H5N1 outbreaks in China and the effect of vaccination on virus evolution.
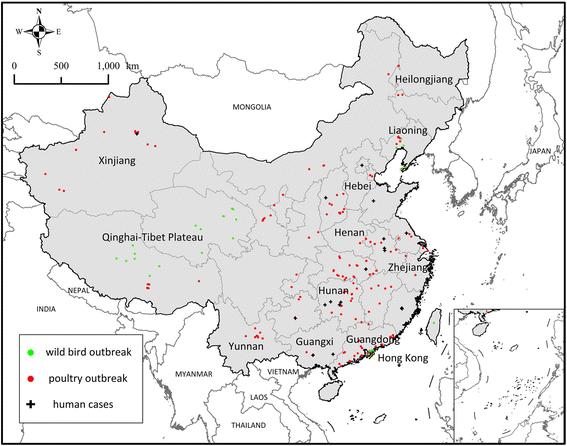
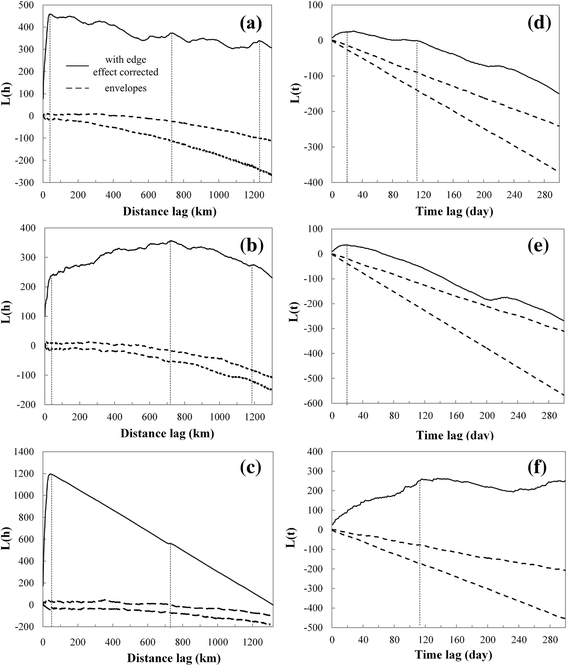
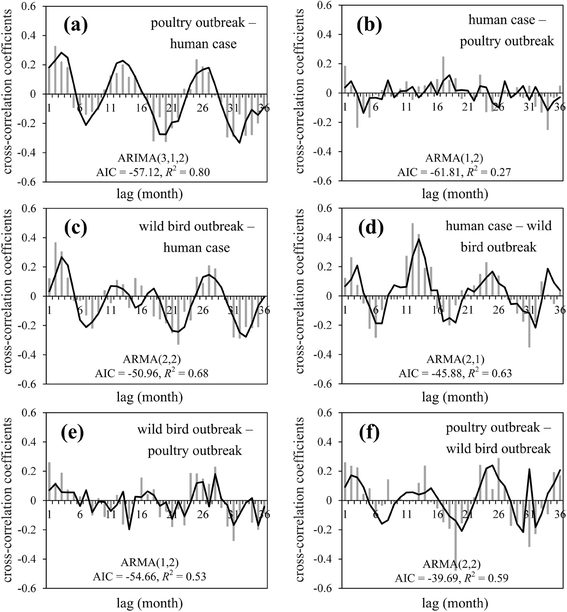
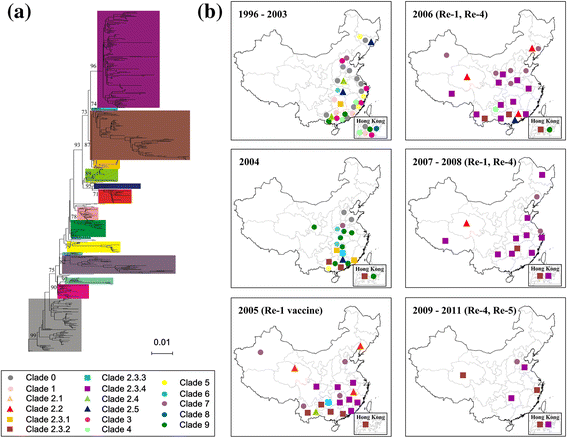
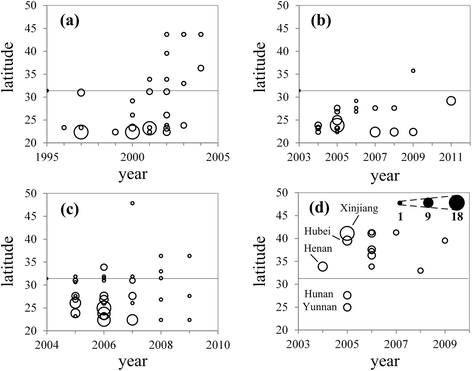
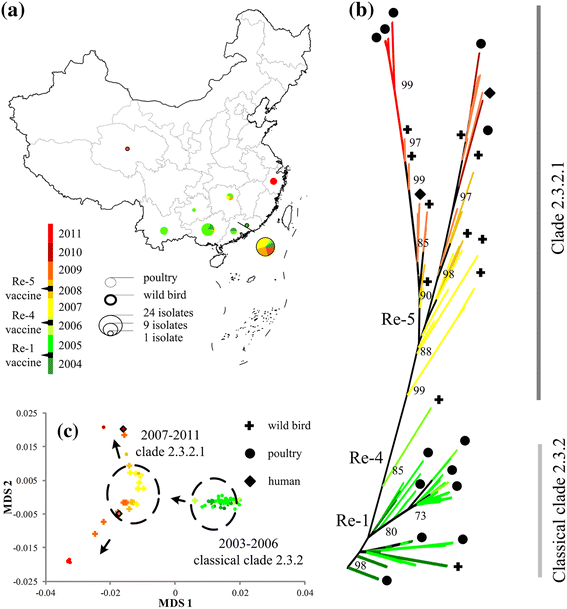
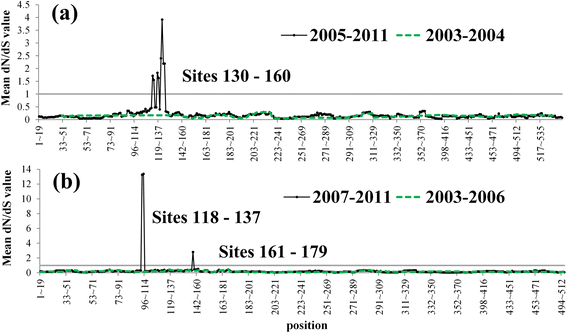
Results: Results showed obvious spatial and temporal clusters of H5N1 outbreaks on different scales, which may have been associated with poultry and wild-bird transmission modes of H5N1 viruses. Lead-lag relationships were found among poultry and wild-bird outbreaks and human cases. Human cases were preceded by poultry outbreaks, and wild-bird outbreaks were led by human cases. Each clade has gained its own unique spatiotemporal and genetic dominance. Genetic diversity of the H5N1 virus decreased significantly between 1996 and 2011; presumably under strong selective pressure of vaccination. Mean evolutionary rates of H5N1 virus increased after vaccination was adopted in China. A clear signature of positively selected sites in the clade 2.3.2 virus was discovered and this may have resulted in the emergence of clade 2.3.2.1.
Conclusions: Our study revealed two different transmission modes of H5N1 viruses in China, and indicated a significant role of poultry in virus dissemination. Furthermore, selective pressure posed by vaccination was found in virus evolution in the country.
Figures
References
-
- Tian H, Zhou S, Dong L, Van Boeckel TP, Pei Y, Wu Q et al. Climate change suggests a shift of H5N1 risk in migratory birds. Ecol Model. 2014, doi:10.1016/j.ecolmodel.2014.08.005.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

