Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury
- PMID: 25887405
- PMCID: PMC4345018
- DOI: 10.1186/s13054-015-0782-3
Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury
Abstract
Introduction: Sepsis refers to severe systemic inflammation leading to acute lung injury (ALI) and death. Introducing novel therapies can reduce the mortality in ALI. Osteopontin (OPN), a secretory glycoprotein produced by immune reactive cells, plays a deleterious role in various inflammatory diseases. However, its role in ALI caused by sepsis remains unexplored. We hypothesize that treatment with an OPN-neutralizing antibody (anti-OPN Ab) protects mice against ALI during sepsis.
Methods: Sepsis was induced in 8-week-old male C57BL/6 mice by cecal ligation and puncture (CLP). Anti-OPN Ab or non-immunized IgG as control, at a dose of 50 μg/mouse, was intravenously injected at the time of CLP. After 20 hours, the expression of OPN and proinflammatory cytokines in tissues and plasma was examined by real-time PCR, Western blot, and ELISA. Plasma levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) and the lung myeloperoxidase (MPO) levels were determined by colorimetric assays. Lung damage and neutrophil infiltrations were determined by histological H&E and Gr-1 staining, respectively. The effect of recombinant mouse OPN (rmOPN) on human neutrophil-like cell (HL-60) migration was performed by Boyden chamber assays and the involvement of intracellular signaling molecules in HL-60 cells was revealed by Western blot.
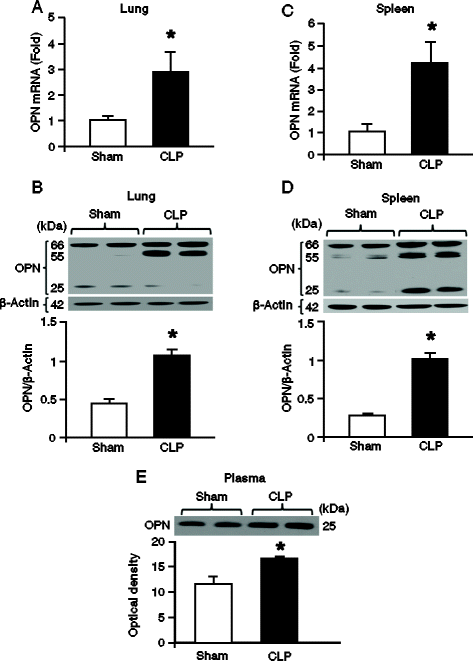
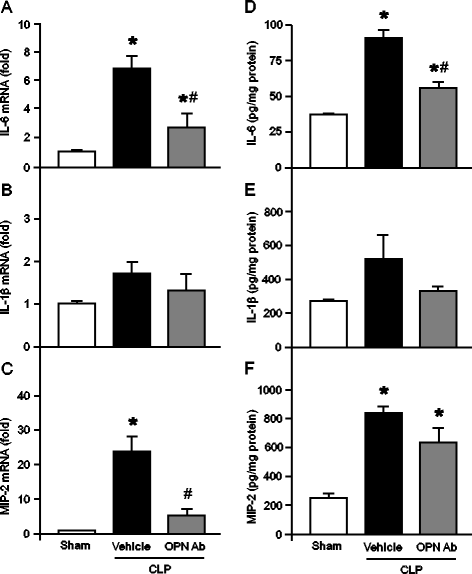
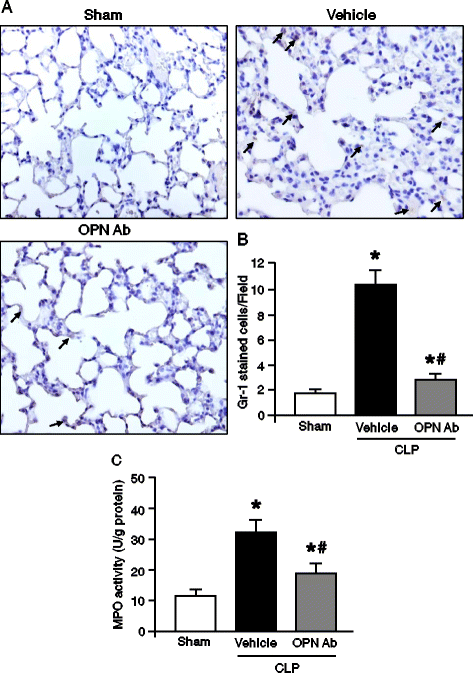
Results: After 20 hours of sepsis, mRNA and protein levels of OPN were significantly induced in lungs, spleen, and plasma. Treatment with an anti-OPN Ab in septic mice significantly reduced the plasma levels of ALT, AST, and LDH, and the proinflammatory cytokines IL-6, IL-1β and the chemokine MIP-2, compared with the vehicle group. Similarly, the lung mRNA and protein expressions of proinflammatory cytokines and chemokine were greatly reduced in anti-OPN Ab-treated animals. The lung histological architecture, MPO and neutrophil infiltration were significantly improved in anti-OPN Ab-treated mice compared with the vehicle animals. Treatment of rmOPN in HL-60 cells significantly increased their migration, in vitro. The neutrophils treated with rmOPN remarkably increased the levels of phospho focal adhesion kinase (pFAK), phospho extracellular signal-regulated kinase (pERK) and phospho p38.
Conclusions: Our findings clearly demonstrate the beneficial outcomes of anti-OPN Ab treatment in protecting against ALI, implicating a novel therapeutic strategy in sepsis.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

