Sleep disordered breathing in mucopolysaccharidosis I: a multivariate analysis of patient, therapeutic and metabolic correlators modifying long term clinical outcome
- PMID: 25887468
- PMCID: PMC4450482
- DOI: 10.1186/s13023-015-0255-4
Sleep disordered breathing in mucopolysaccharidosis I: a multivariate analysis of patient, therapeutic and metabolic correlators modifying long term clinical outcome
Abstract
Background: The lysosomal storage disorder, mucopolysaccharidosis I (MPS I), commonly manifests with upper airway obstruction and sleep disordered breathing (SDB). The success of current therapies, including haematopoietic stem cell transplantation (HSCT) and enzyme replacement therapy (ERT) may be influenced by a number of factors and monitored using biomarkers of metabolic correction. We describe the pattern of SDB seen in the largest MPS I cohort described to date and determine therapies and biomarkers influencing the severity of long-term airway disease.
Methods: Therapeutic, clinical and biomarker data, including longitudinal outcome parameters from 150 sleep oximetry studies were collected in 61 MPS I (44 Hurler, 17 attenuated) patients between 6 months pre to 16 years post-treatment (median follow-up 22 months). The presence and functional nature of an immune response to ERT was determined using ELISA and a cellular uptake inhibition assay. Multivariate analysis was performed to determine significant correlators of airway disease.
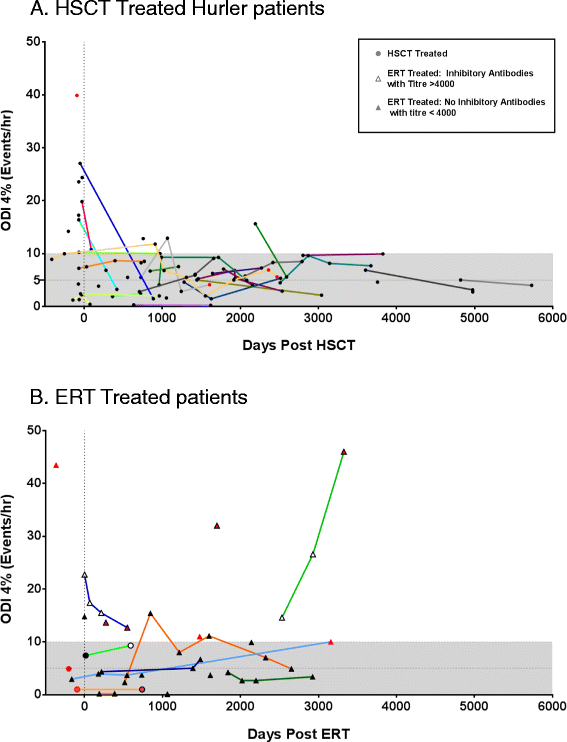
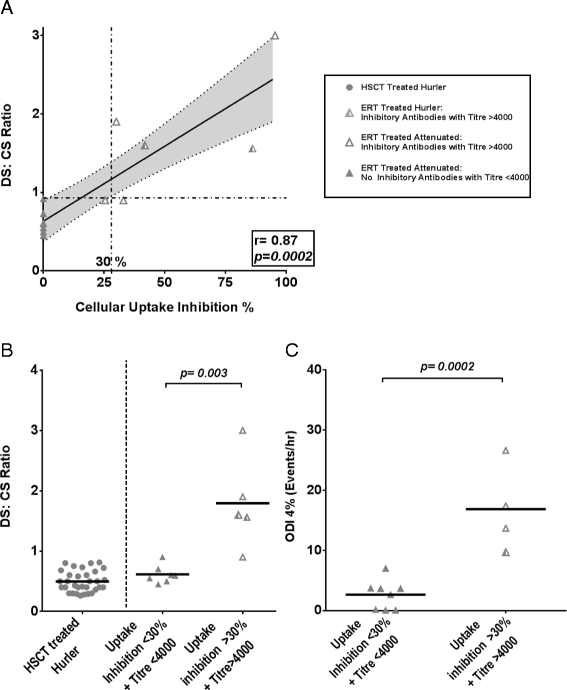
Results: The incidence of SDB in our cohort is 68%, while 16% require therapeutic intervention for airway obstruction. A greater rate of progression (73%) and requirement for intervention is seen amongst ERT patients in contrast to HSCT treated individuals (24%). Multivariate analysis identifies poorer metabolic clearance, as measured by a rise in the biomarker urinary dermatan sulphate: chondroitin sulphate (DS:CS) ratio, as a significant correlator of increased presence and severity of SDB in MPS I patients (p = 0.0017, 0.008). Amongst transplanted Hurler patients, delivered enzyme (leukocyte iduronidase) at one year is significantly raised in those without SDB (p = 0.004). Cellular uptake inhibitory antibodies in ERT treated patients correlate with reduced substrate clearance and occurrence of severe SDB (p = 0.001).
Conclusion: We have identified biochemical and therapeutic factors modifying airway disease across the phenotypic spectrum in MPS I. Interventions maximising substrate reduction correlate with improved long-term SDB, while inhibitory antibodies impact on biochemical and clinical outcomes. Monitoring and tolerisation strategies should be re-evaluated to improve detection and minimise the inhibitory antibody response to ERT in MPS I and other lysosomal storage diseases. Future studies should consider the use of sleep disordered breathing as an objective parameter of clinical and metabolic improvement.
Figures

References
-
- de Ru MH, Boelens JJ, Das AM, Jones SA, van der Lee JH, Mahlaoui N, et al. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure. Orphanet J Rare Dis. 2011;6:55. doi: 10.1186/1750-1172-6-55. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

