Automatic segmentation of deep intracerebral electrodes in computed tomography scans
- PMID: 25887573
- PMCID: PMC4393625
- DOI: 10.1186/s12859-015-0511-6
Automatic segmentation of deep intracerebral electrodes in computed tomography scans
Abstract
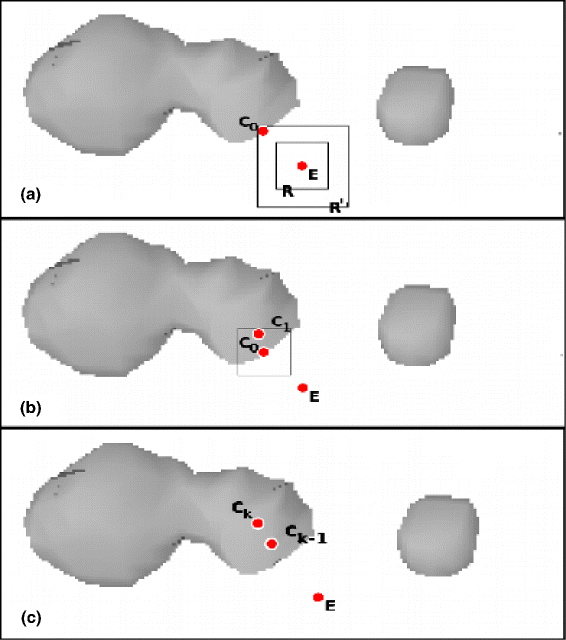
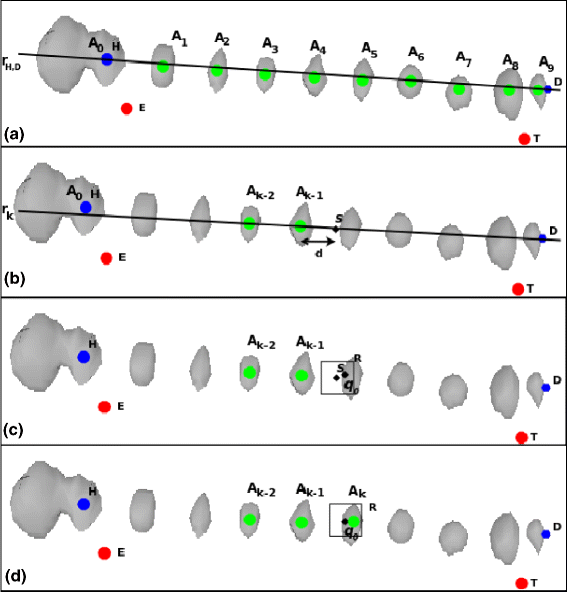
Background: Invasive monitoring of brain activity by means of intracerebral electrodes is widely practiced to improve pre-surgical seizure onset zone localization in patients with medically refractory seizures. Stereo-Electroencephalography (SEEG) is mainly used to localize the epileptogenic zone and a precise knowledge of the location of the electrodes is expected to facilitate the recordings interpretation and the planning of resective surgery. However, the localization of intracerebral electrodes on post-implant acquisitions is usually time-consuming (i.e., manual segmentation), it requires advanced 3D visualization tools, and it needs the supervision of trained medical doctors in order to minimize the errors. In this paper we propose an automated segmentation algorithm specifically designed to segment SEEG contacts from a thresholded post-implant Cone-Beam CT volume (0.4 mm, 0.4 mm, 0.8 mm). The algorithm relies on the planned position of target and entry points for each electrode as a first estimation of electrode axis. We implemented the proposed algorithm into DEETO, an open source C++ prototype based on ITK library.
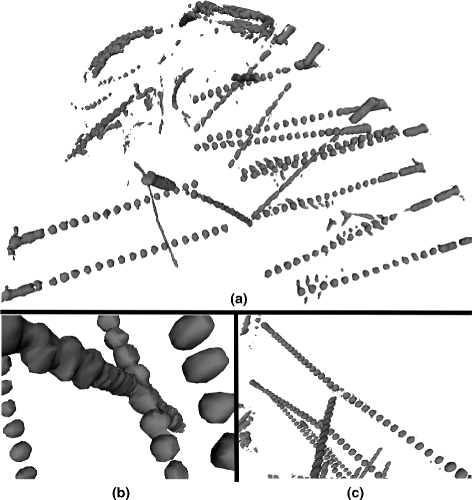
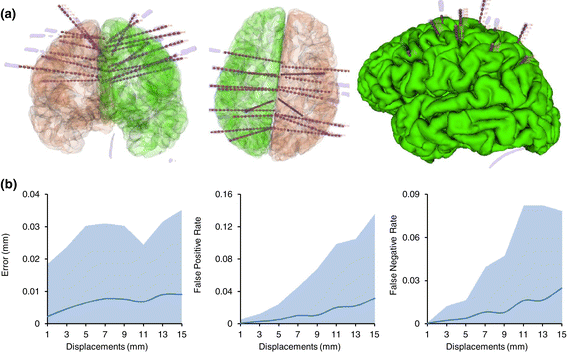
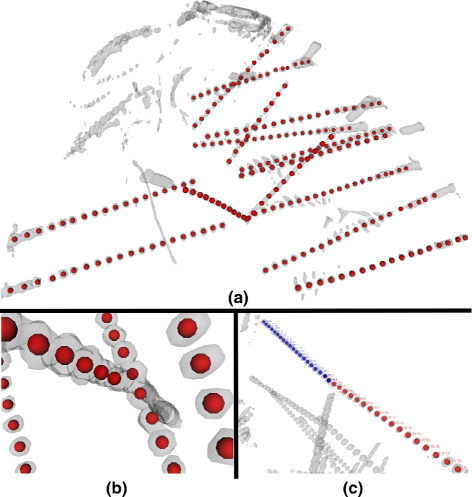
Results: We tested our implementation on a cohort of 28 subjects in total. The experimental analysis, carried out over a subset of 12 subjects (35 multilead electrodes; 200 contacts) manually segmented by experts, show that the algorithm: (i) is faster than manual segmentation (i.e., less than 1s/subject versus a few hours) (ii) is reliable, with an error of 0.5 mm ± 0.06 mm, and (iii) it accurately maps SEEG implants to their anatomical regions improving the interpretability of electrophysiological traces for both clinical and research studies. Moreover, using the 28-subject cohort we show here that the algorithm is also robust (error < 0.005 mm) against deep-brain displacements (< 12 mm) of the implanted electrode shaft from those planned before surgery.
Conclusions: Our method represents, to the best of our knowledge, the first automatic algorithm for the segmentation of SEEG electrodes. The method can be used to accurately identify the neuroanatomical loci of SEEG electrode contacts by a non-expert in a fast and reliable manner.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

