I can see CRISPR now, even when phage are gone: a view on alternative CRISPR-Cas functions from the prokaryotic envelope
- PMID: 25887612
- PMCID: PMC4414917
- DOI: 10.1097/QCO.0000000000000154
I can see CRISPR now, even when phage are gone: a view on alternative CRISPR-Cas functions from the prokaryotic envelope
Abstract
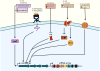
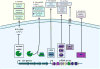
Purpose of review: CRISPR-Cas systems are prokaryotic immune systems against invading nucleic acids that adapt as new environmental threats arise. There are emerging examples of CRISPR-Cas functions in bacterial physiology beyond their role in adaptive immunity. This highlights the poorly understood, but potentially common, moonlighting functions of these abundant systems. We propose that these noncanonical CRISPR-Cas activities have evolved to respond to stresses at the cell envelope.
Recent findings: Here, we discuss recent literature describing the impact of the extracellular environment on the regulation of CRISPR-Cas systems, and the influence of CRISPR-Cas activity on bacterial physiology. These described noncanonical CRISPR-Cas functions allow the bacterial cell to respond to the extracellular environment, primarily through changes in envelope physiology.
Summary: This review discusses the expanding noncanonical functions of CRISPR-Cas systems, including their roles in virulence, focusing mainly on their relationship to the cell envelope. We first examine the effects of the extracellular environment on regulation of CRISPR-Cas components, and then discuss the impact of CRISPR-Cas systems on bacterial physiology, concentrating on their roles in influencing interactions with the environment including host organisms.
Conflict of interest statement
Figures
Similar articles
-
[The functional aspects of bacterial CRISPR-cas systems and interactions between phages and its bacterial hosts--a review].Wei Sheng Wu Xue Bao. 2015 Mar 4;55(3):251-7. Wei Sheng Wu Xue Bao. 2015. PMID: 26065266 Review. Chinese.
-
The roles of CRISPR-Cas systems in adaptive immunity and beyond.Curr Opin Immunol. 2015 Feb;32:36-41. doi: 10.1016/j.coi.2014.12.008. Epub 2015 Jan 6. Curr Opin Immunol. 2015. PMID: 25574773 Review.
-
Role of CRISPR/cas system in the development of bacteriophage resistance.Adv Virus Res. 2012;82:289-338. doi: 10.1016/B978-0-12-394621-8.00011-X. Adv Virus Res. 2012. PMID: 22420856 Review.
-
CRISPR-Cas systems: new players in gene regulation and bacterial physiology.Front Cell Infect Microbiol. 2014 Apr 4;4:37. doi: 10.3389/fcimb.2014.00037. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24772391 Free PMC article. Review.
-
To acquire or resist: the complex biological effects of CRISPR-Cas systems.Trends Microbiol. 2014 Apr;22(4):218-25. doi: 10.1016/j.tim.2014.01.007. Epub 2014 Feb 26. Trends Microbiol. 2014. PMID: 24582529 Review.
Cited by
-
Exposure to 1-Butanol Exemplifies the Response of the Thermoacidophilic Archaeon Sulfolobus acidocaldarius to Solvent Stress.Appl Environ Microbiol. 2021 May 11;87(11):e02988-20. doi: 10.1128/AEM.02988-20. Print 2021 May 11. Appl Environ Microbiol. 2021. PMID: 33741627 Free PMC article.
-
Functional Analysis of Bacteriophage Immunity through a Type I-E CRISPR-Cas System in Vibrio cholerae and Its Application in Bacteriophage Genome Engineering.J Bacteriol. 2015 Nov 23;198(3):578-90. doi: 10.1128/JB.00747-15. Print 2016 Feb 1. J Bacteriol. 2015. PMID: 26598368 Free PMC article.
-
Importance of mobile genetic element immunity in numerically abundant Trichodesmium clades.ISME Commun. 2023 Feb 23;3(1):15. doi: 10.1038/s43705-023-00214-y. ISME Commun. 2023. PMID: 36823453 Free PMC article.
-
CRISPR/Cas System and Factors Affecting Its Precision and Efficiency.Front Cell Dev Biol. 2021 Nov 24;9:761709. doi: 10.3389/fcell.2021.761709. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34901007 Free PMC article. Review.
-
Overview of CRISPR-Cas9 Biology.Cold Spring Harb Protoc. 2016 Dec 1;2016(12):pdb.top088849. doi: 10.1101/pdb.top088849. Cold Spring Harb Protoc. 2016. PMID: 27934695 Free PMC article.
References
-
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science. 2007;315(5819):1709–12. - PubMed
-
- Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482(7385):331–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

