Neutral lipid alterations in human herpesvirus 8-infected HUVEC cells and their possible involvement in neo-angiogenesis
- PMID: 25887745
- PMCID: PMC4384337
- DOI: 10.1186/s12866-015-0415-7
Neutral lipid alterations in human herpesvirus 8-infected HUVEC cells and their possible involvement in neo-angiogenesis
Abstract
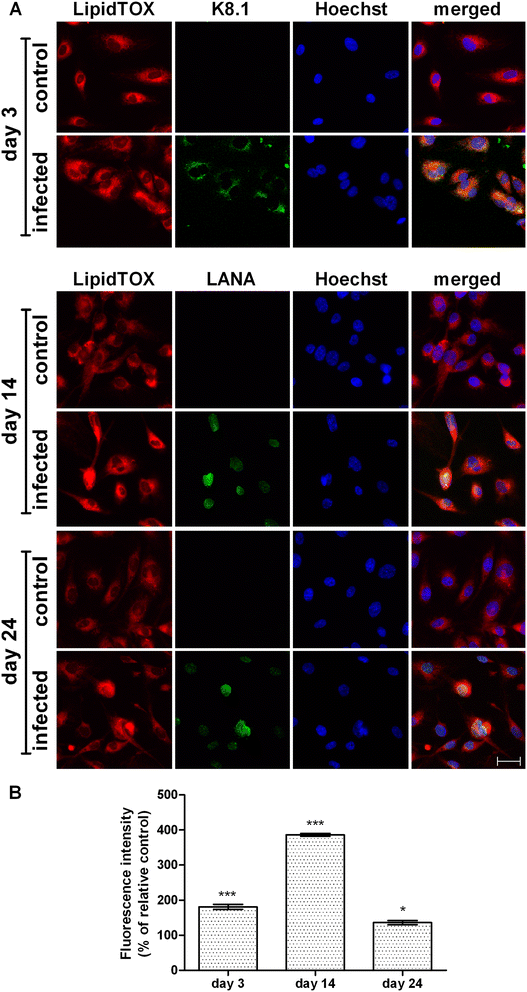
Background: Human Herpesvirus 8 (HHV8), the causative agent of Kaposi's sarcoma, induces an intense modification of lipid metabolism and enhances the angiogenic process in endothelial cells. In the present study, neutral lipid (NL) metabolism and angiogenesis were investigated in HHV8-infected HUVEC cells. The viral replication phases were verified by rtPCR and also by K8.1 and LANA immunostaining.
Results: Lipid droplets (Nile Red) were higher in all phases and NL staining (LipidTOX) combined with viral-antigen detection (immunofluorescence) demonstrated a NL content increase in infected cells. In particular, triglyceride synthesis increases in the lytic phase, whereas cholesteryl ester synthesis rises in the latent one. Moreover, the inhibition of cholesterol esterification reduces neo-tubule formation mainly in latently infected cells.
Conclusions: We suggest that a reprogramming of cholesteryl ester metabolism is involved in regulating neo-angiogenesis in HHV8-infected cells and plays a likely role in the high metastatic potential of derived-tumours.
Figures




References
-
- Hengge UR, Ruzicka T, Tyring SK, Stuschke M, Roggendorf M, Schwartz RA, et al. Update on Kaposi's sarcoma and other HHV8 associated diseases. Part 1: epidemiology, environmental predispositions, clinical manifestations, and therapy. Lancet Infect Dis. 2002;2(5):281–292. doi: 10.1016/S1473-3099(02)00263-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

