Emerging EPO and EPO receptor regulators and signal transducers
- PMID: 25887776
- PMCID: PMC4458796
- DOI: 10.1182/blood-2014-11-575357
Emerging EPO and EPO receptor regulators and signal transducers
Abstract
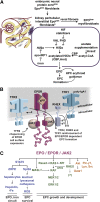
As essential mediators of red cell production, erythropoietin (EPO) and its cell surface receptor (EPO receptor [EPOR]) have been intensely studied. Early investigations defined basic mechanisms for hypoxia-inducible factor induction of EPO expression, and within erythroid progenitors EPOR engagement of canonical Janus kinase 2/signal transducer and activator of transcription 5 (JAK2/STAT5), rat sarcoma/mitogen-activated protein kinase/extracellular signal-regulated kinase (RAS/MEK/ERK), and phosphatidylinositol 3-kinase (PI3K) pathways. Contemporary genetic, bioinformatic, and proteomic approaches continue to uncover new clinically relevant modulators of EPO and EPOR expression, and EPO's biological effects. This Spotlight review highlights such factors and their emerging roles during erythropoiesis and anemia.
© 2015 by The American Society of Hematology.
Figures
References
-
- Reissmann KR. Studies on the mechanism of erythropoietic stimulation in parabiotic rats during hypoxia. Blood. 1950;5(4):372–380. - PubMed
-
- Miyake T, Kung CK, Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977;252(15):5558–5564. - PubMed
-
- Jacobs K, Shoemaker C, Rudersdorf R, et al. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature. 1985;313(6005):806–810. - PubMed
-
- Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology and the American Society of Clinical Oncology Practice Guideline Update Committee. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 2010;116(20):4045–4059. - PubMed
-
- D’Andrea AD, Lodish HF, Wong GG. Expression cloning of the murine erythropoietin receptor. Cell. 1989;57(2):277–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

