Targeting of folate receptor β on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells
- PMID: 25887778
- PMCID: PMC4447861
- DOI: 10.1182/blood-2014-11-612721
Targeting of folate receptor β on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells
Abstract
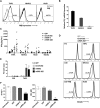
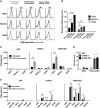
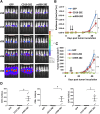
T cells expressing a chimeric antigen receptor (CAR) can produce dramatic results in lymphocytic leukemia patients; however, therapeutic strategies for myeloid leukemia remain limited. Folate receptor β (FRβ) is a myeloid-lineage antigen expressed on 70% of acute myeloid leukemia (AML) patient samples. Here, we describe the development and evaluation of the first CARs specific for human FRβ (m909) in vitro and in vivo. m909 CAR T cells exhibited selective activation and lytic function against engineered C30-FRβ as well as endogenous FRβ(+) AML cell lines in vitro. In mouse models of human AML, m909 CAR T cells mediated the regression of engrafted FRβ(+) THP1 AML in vivo. In addition, we demonstrated that treatment of AML with all-trans retinoic acid (ATRA) enhanced FRβ expression, resulting in improved immune recognition by m909 CAR T cells. Because many cell surface markers are shared between AML blasts and healthy hematopoietic stem and progenitor cells (HSCs), we evaluated FRβ expression and recognition of HSCs by CAR T cells. m909 CAR T cells were not toxic against healthy human CD34(+) HSCs in vitro. Our results indicate that FRβ is a promising target for CAR T-cell therapy of AML, which may be augmented by combination with ATRA.
Figures



References
-
- Roboz GJ. Current treatment of acute myeloid leukemia. Curr Opin Oncol. 2012;24(6):711–719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

