Expression of catalase and retinoblastoma-related protein genes associates with cell death processes in Scots pine zygotic embryogenesis
- PMID: 25887788
- PMCID: PMC4396594
- DOI: 10.1186/s12870-015-0462-0
Expression of catalase and retinoblastoma-related protein genes associates with cell death processes in Scots pine zygotic embryogenesis
Abstract
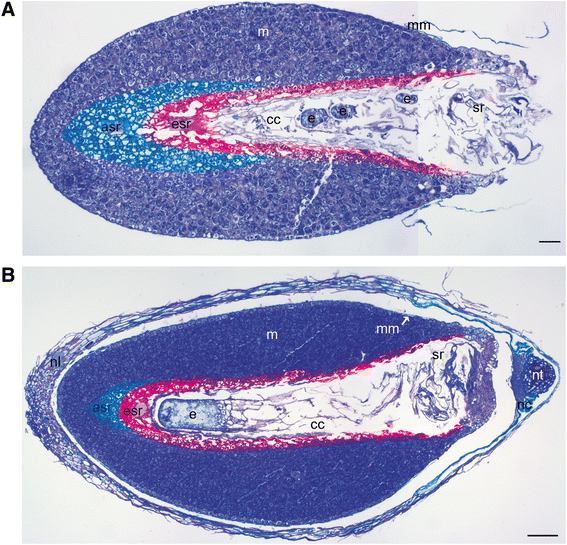
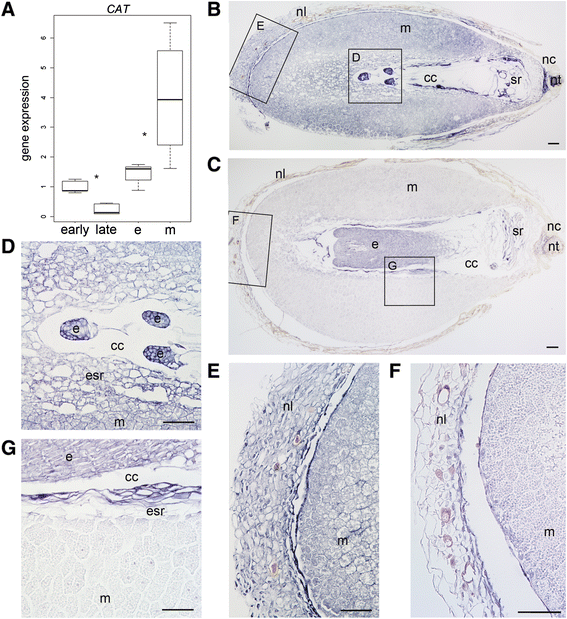
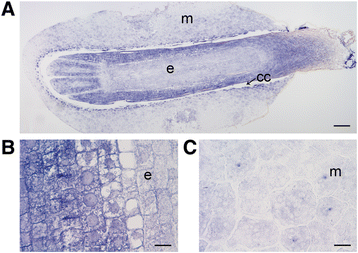
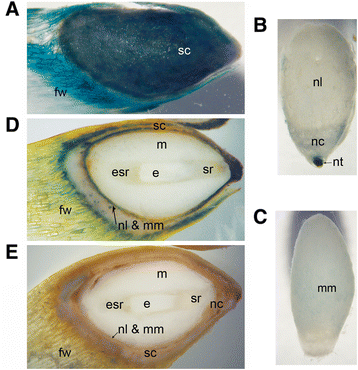
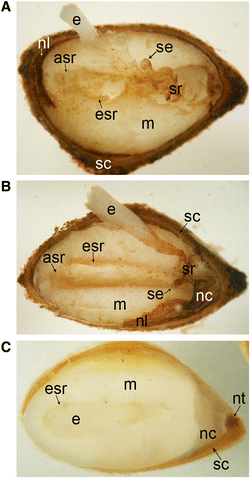
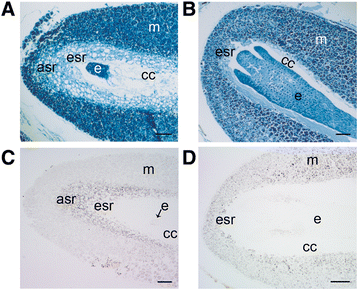
Background: The cell cycle and cellular oxidative stress responses are tightly controlled for proper growth and development of Scots pine (Pinus sylvestris L.) seed. Programmed cell death (PCD) is an integral part of the embryogenesis during which megagametophyte cells in the embryo surrounding region (ESR) and cells in the nucellar layers face death. In the present study, we show both the tissue and developmental stage specific expression of the genes encoding the autophagy related ATG5, catalase (CAT), and retinoblastoma related protein (RBR) as well as the connection between the gene expressions and cell death programs.
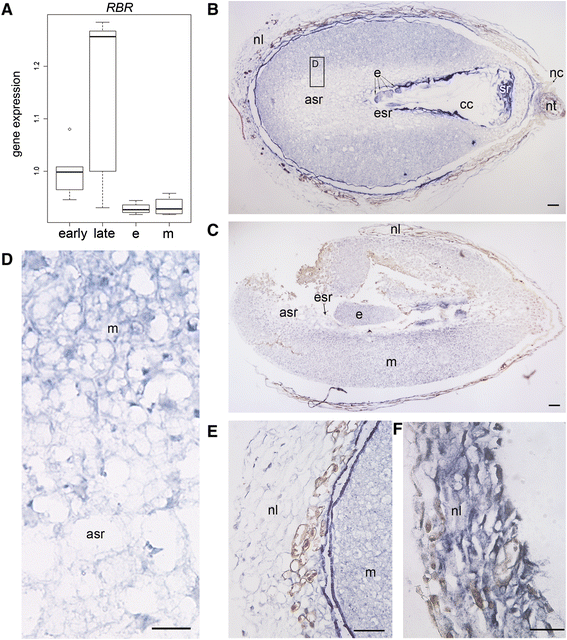
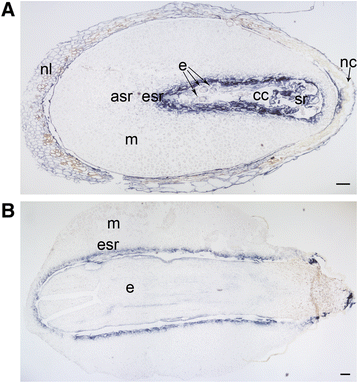
Results: We found strong CAT expression in the cells of the developing embryo throughout the embryogenesis as well as in the cells of the megagametophyte and the nucellar layers at the early embryogeny. The CAT expression was found to overlap with both the ATG5 expression and hydrogen peroxide localization. At the late embryogeny, CAT expression diminished in the dying cells of the nucellar layers as well as in megagametophyte cells, showing the first signs of incipient cell death. Accumulation of starch and minor RBR expression were characteristic of megagametophyte cells in the ESR, whereas strong RBR expression was found in the cells of the nucellar layers at the late embryogeny.
Conclusions: Our results suggest that ATG5, CAT, and RBR are involved in the Scots pine embryogenesis and cell death processes. CAT seems to protect cells against hydrogen peroxide accumulation and oxidative stress related cell death especially during active metabolism. The opposite expression of RBR in the ESR and nucellar layers alongside morphological characteristics emphasizes the different type of the cell death processes in these tissues. Furthermore, the changes in ATG5 and RBR expressions specifically in the megagametophyte cells dying by necrotic cell death suggest the genetic regulation of developmental necrosis in Scots pine embryogenesis.
Figures
Similar articles
-
Moderate stress responses and specific changes in polyamine metabolism characterize Scots pine somatic embryogenesis.Tree Physiol. 2016 Mar;36(3):392-402. doi: 10.1093/treephys/tpv136. Epub 2016 Jan 19. Tree Physiol. 2016. PMID: 26786537 Free PMC article.
-
One tissue, two fates: different roles of megagametophyte cells during Scots pine embryogenesis.J Exp Bot. 2009;60(4):1375-86. doi: 10.1093/jxb/erp020. Epub 2009 Feb 26. J Exp Bot. 2009. PMID: 19246593 Free PMC article.
-
Microscopical Detection of Cell Death Processes During Scots Pine Zygotic Embryogenesis.Methods Mol Biol. 2020;2122:223-237. doi: 10.1007/978-1-0716-0342-0_16. Methods Mol Biol. 2020. PMID: 31975306
-
Roles of plant retinoblastoma protein: cell cycle and beyond.EMBO J. 2020 Oct 1;39(19):e105802. doi: 10.15252/embj.2020105802. Epub 2020 Aug 31. EMBO J. 2020. PMID: 32865261 Free PMC article. Review.
-
Emerging roles of RETINOBLASTOMA-RELATED proteins in evolution and plant development.Trends Plant Sci. 2012 Mar;17(3):139-48. doi: 10.1016/j.tplants.2011.12.001. Epub 2012 Jan 10. Trends Plant Sci. 2012. PMID: 22240181 Review.
Cited by
-
The catalase gene family in cucumber: genome-wide identification and organization.Genet Mol Biol. 2016 Jul-Sep;39(3):408-15. doi: 10.1590/1678-4685-GMB-2015-0192. Epub 2016 Jul 25. Genet Mol Biol. 2016. PMID: 27560990 Free PMC article.
-
Scots pine aminopropyltransferases shed new light on evolution of the polyamine biosynthesis pathway in seed plants.Ann Bot. 2018 May 11;121(6):1243-1256. doi: 10.1093/aob/mcy012. Ann Bot. 2018. PMID: 29462244 Free PMC article.
-
Catalase (CAT) Gene Family in Rapeseed (Brassica napus L.): Genome-Wide Analysis, Identification, and Expression Pattern in Response to Multiple Hormones and Abiotic Stress Conditions.Int J Mol Sci. 2021 Apr 20;22(8):4281. doi: 10.3390/ijms22084281. Int J Mol Sci. 2021. PMID: 33924156 Free PMC article.
-
Changing winter climate and snow conditions induce various transcriptional stress responses in Scots pine seedlings.Front Plant Sci. 2022 Dec 9;13:1050903. doi: 10.3389/fpls.2022.1050903. eCollection 2022. Front Plant Sci. 2022. PMID: 36570907 Free PMC article.
-
Analysis of CAT Gene Family and Functional Identification of OsCAT3 in Rice.Genes (Basel). 2023 Jan 4;14(1):138. doi: 10.3390/genes14010138. Genes (Basel). 2023. PMID: 36672879 Free PMC article.
References
-
- Lam E. Programmed cell death in plants: orchestrating an intrinsic suicide program within walls. Crit Rev Plant Sci. 2008;27(6):413–23. doi: 10.1080/07352680802467744. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

