An automated image analysis method to measure regularity in biological patterns: a case study in a Drosophila neurodegenerative model
- PMID: 25887846
- PMCID: PMC4367968
- DOI: 10.1186/s13024-015-0005-z
An automated image analysis method to measure regularity in biological patterns: a case study in a Drosophila neurodegenerative model
Abstract
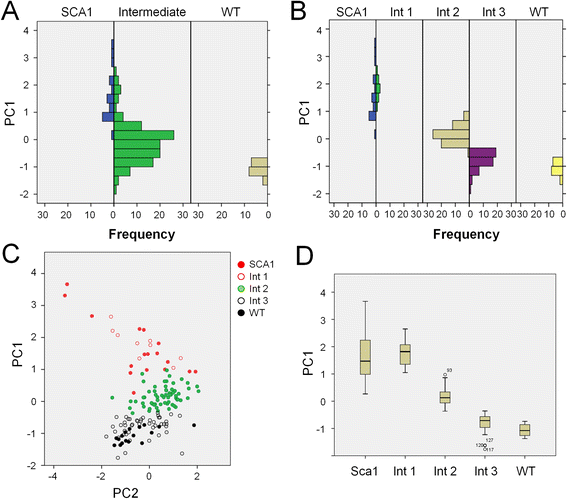
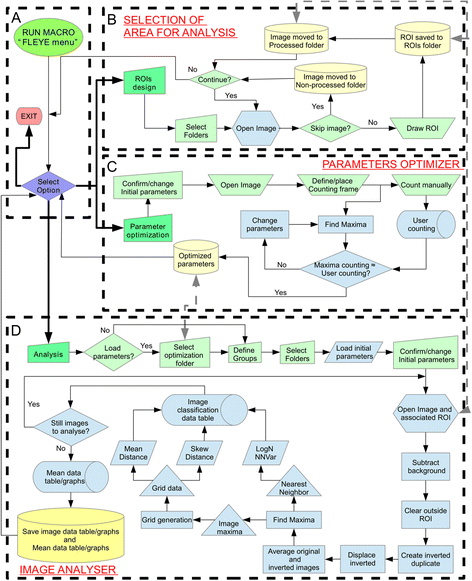
The fruitfly compound eye has been broadly used as a model for neurodegenerative diseases. Classical quantitative techniques to estimate the degeneration level of an eye under certain experimental conditions rely either on time consuming histological techniques to measure retinal thickness, or pseudopupil visualization and manual counting. Alternatively, visual examination of the eye surface appearance gives only a qualitative approximation provided the observer is well-trained. Therefore, there is a need for a simplified and standardized analysis of fruitfly eye degeneration extent for both routine laboratory use and for automated high-throughput analysis. We have designed the freely available ImageJ plugin FLEYE, a novel and user-friendly method for quantitative unbiased evaluation of neurodegeneration levels based on the acquisition of fly eye surface pictures. The incorporation of automated image analysis tools and a classification algorithm sustained on a built-in statistical model allow the user to quickly analyze large sample size data with reliability and robustness. Pharmacological screenings or genetic studies using the Drosophila retina as a model system may benefit from our method, because it can be easily implemented in a fully automated environment. In addition, FLEYE can be trained to optimize the image detection capabilities, resulting in a versatile approach to evaluate the pattern regularity of other biological or non-biological samples and their experimental or pathological disruption.
Figures





References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

