Long-term hazard of recurrence in HER2+ breast cancer patients untreated with anti-HER2 therapy
- PMID: 25888246
- PMCID: PMC4423419
- DOI: 10.1186/s13058-015-0568-1
Long-term hazard of recurrence in HER2+ breast cancer patients untreated with anti-HER2 therapy
Abstract
Introduction: Worldwide, many patients with HER2+ (human epidermal growth factor receptor 2-positive) early breast cancer (BC) do not receive adjuvant trastuzumab. Hazards of recurrence of these patients with respect to hormone receptor status of the primary tumor have not been described.
Methods: Using data from 1,260 patients randomized to placebo in the adjuvant TEACH trial, we report 10-year annual hazards of recurrence in HER2+ patients not treated with anti-HER2 therapy.
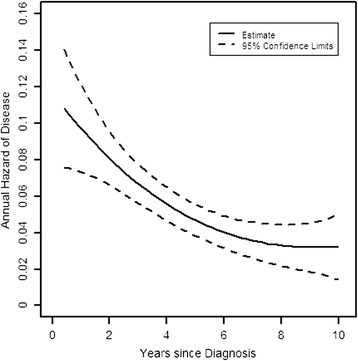
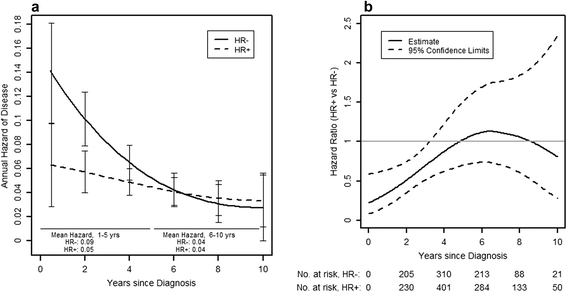
Results: Disease-free survival (DFS) was 75% after 5 and 61% after 10 years, respectively. Patients with HER2+ hormone receptor-positive (HR+ (hormone receptor-positive); ER+ (estrogen receptor-positive) or PR+ (progesterone receptor-positive)) disease had a significantly better DFS than patients with HER2+ HR- (ER-/PR-) disease (hazard ratio 0.72, P=0.02). This difference was explainable by a significantly higher hazard of recurrence in years 1 to 5 in HER2+ HR- compared to HER2+ HR+ patients, with a mean risk of recurrence of 9%/year for HR- versus 5%/year in HR+ patients (hazard ratio 0.59, P=0.002 for years 1 to 5). The high early risk of recurrence of HER2+ HR- patients declined sharply over time, so that it was similar to that seen in HER2+ HR+ patients in years 6 to 10 (hazard ratio 0.97, P=0.92 for years 6 to 10).
Conclusions: Our results show that outcomes in HER2+ patients with early BC not receiving anti-HER2 therapy strongly depend on HR expression. The very high early risk of relapse seen in HER2+ HR- patients is particularly relevant in health care settings with limited access to adjuvant anti-HER2 treatment. The event rates shown for subpopulations of HER2+ BC patients suggest that in resource-constrained environments patients with HER2+ HR- early BC should be prioritized for consideration of adjuvant anti-HER2 therapy.
Figures

References
-
- Hosmer DW, Lemeshow S. Applied survival analysis: regression modeling of time to event data, vol. 1. 1st ed. New York: Wiley & Sons; 1999.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

