Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation
- PMID: 25888259
- PMCID: PMC5811222
- DOI: 10.1016/j.immuni.2015.03.008
Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation
Abstract
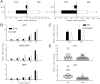
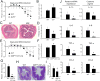
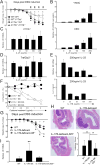
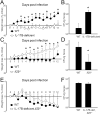
The interleukin-17 (IL-17) family of cytokines has emerged as a critical player in inflammatory diseases. Among them, IL-25 has been shown to be important in allergic inflammation and protection against parasitic infection. Here we have demonstrated that IL-17B, a poorly understood cytokine, functions to inhibit IL-25-driven inflammation. IL-17B and IL-25, both binding to the interleukin-17 receptor B (IL-17RB), were upregulated in their expression after acute colonic inflammation. Individual inhibition of these cytokines revealed opposing functions in colon inflammation: IL-25 was pathogenic but IL-17B was protective. Similarly opposing phenotypes were observed in Citrobacter rodentium infection and allergic asthma. Moreover, IL-25 was found to promote IL-6 production from colon epithelial cells, which was inhibited by IL-17B. Therefore, our data demonstrate that IL-17B is an anti-inflammatory cytokine in the IL-17 family.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Comment in
-
Cytokines: An antagonistic family member.Nat Rev Immunol. 2015 May;15(5):266-7. doi: 10.1038/nri3854. Nat Rev Immunol. 2015. PMID: 25907456 No abstract available.
References
-
- Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nature medicine. 2000;6:583–588. - PubMed
-
- Buning C, Genschel J, Weltrich R, Lochs H, Schmidt H. The interleukin-25 gene located in the inflammatory bowel disease (IBD) 4 region: no association with inflammatory bowel disease. European journal of immunogenetics: official journal of the British Society for Histocompatibility and Immunogenetics. 2003;30:329–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

