Efficacy of bevacizumab and chemotherapy in the first-line treatment of metastatic colorectal cancer: broadening KRAS-focused clinical view
- PMID: 25888291
- PMCID: PMC4376345
- DOI: 10.1186/s12876-015-0266-6
Efficacy of bevacizumab and chemotherapy in the first-line treatment of metastatic colorectal cancer: broadening KRAS-focused clinical view
Abstract
Background: The aim of the present retrospective study was to analyze clinical outcome and risk factors associated with treatment outcomes according to KRAS status in patient with metastatic colorectal cancer (mCRC) treated with bevacizumab (bev) plus chemotherapy in the first-line setting.
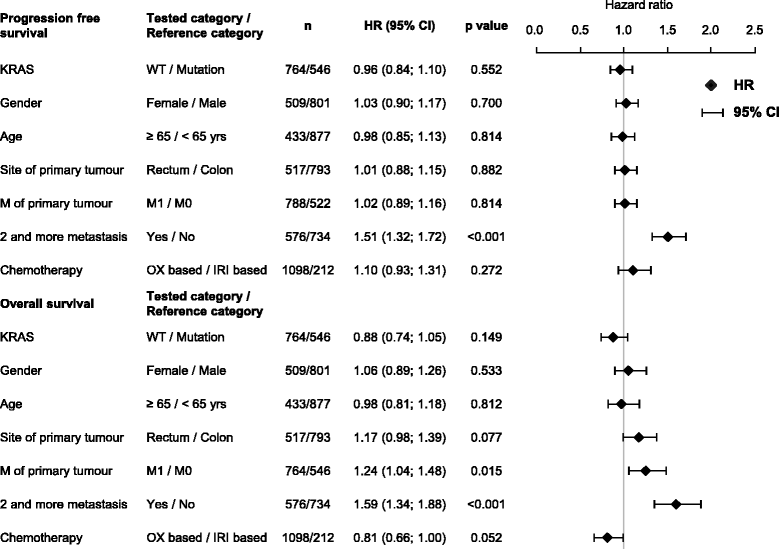
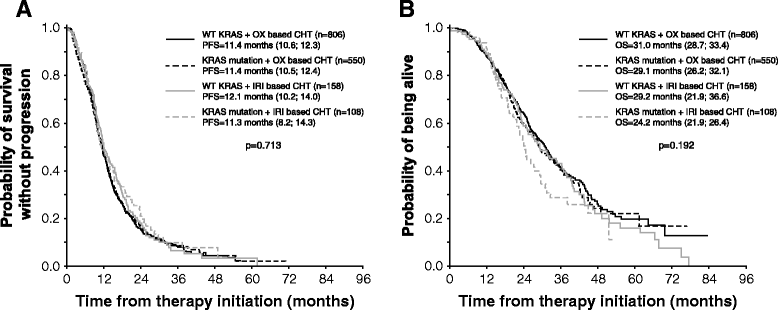
Methods: We performed observational study on 1622 patients with mCRC treated with bev plus oxaliplatin- or irinotecan-based chemotherapy, and correlated treatment outcomes with KRAS mutation status. The primary endpoint was progression-free survival (PFS) and additionally overall survival (OS). Adverse events of bevacizumab and risk factors including location of metastases were evaluated.
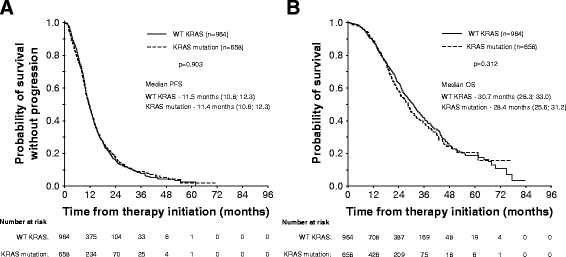
Results: Mutation in KRAS was present in 40.6% of mCRC cases. The median PFS in patients with wild-type KRAS (wtKRAS) vs mutant KRAS was 11.5 vs 11.4 months, respectively. The median OS was 30.7 vs 28.4 months (p = 0.312). Patients with KRAS mutation had lung metastases more frequently than wtKRAS individuals (32.0% vs 23.8%; p = 0.001). We observed no difference in clinical outcome between hepatic and extrahepatic metastatic disease.
Conclusion: KRAS mutation does not interfere with clinical benefit from first-line treatment with bevacizumab plus chemotherapy in mCRC patients.
Figures

References
-
- Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, et al. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55:4575–80. - PubMed
-
- Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–8. - PubMed
-
- Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44. doi: 10.1200/JCO.2006.09.6305. - DOI - PubMed
-
- Rak J, Yu JL, Kerbel RS, Coomber BL. What do oncogenic mutations have to do with angiogenesis/vascular dependence of tumors? Cancer Res. 2002;62:1931–4. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

