Does transient cART started during primary HIV infection undermine the long-term immunologic and virologic response on cART resumption?
- PMID: 25888386
- PMCID: PMC4403722
- DOI: 10.1186/s12879-015-0892-1
Does transient cART started during primary HIV infection undermine the long-term immunologic and virologic response on cART resumption?
Abstract
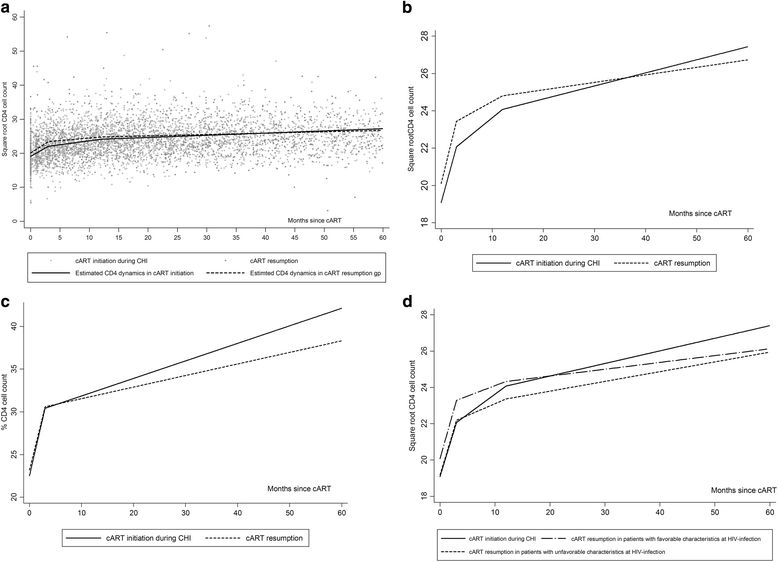
Background: We explored the impact of transient cART started during the primary HIV-infection (PHI) on the long-term immunologic and virologic response on cART resumption, by comparison with treatment initiation during the chronic phase of HIV infection (CHI).
Methods: We analyzed data on 1450 patients enrolled during PHI in the ANRS PRIMO cohort between 1996 and 2013. "Treatment resumption" was defined as at least 3 months of resumed treatment following interruption of at least 1 month of treatment initiated during PHI. "Treatment initiation during CHI" was defined as cART initiated ≥6 months after PHI. The virologic response to resumed treatment and to treatment initiated during CHI was analyzed with survival models. The CD4 cell count dynamics was modeled with piecewise linear mixed models.
Results: 136 patients who resumed cART for a median (IQR) of 32 (18-51) months were compared with 377 patients who started cART during CHI for a median of 45 (22-57) months. Most patients (97%) achieved HIV-RNA <50 cp/mL after similar times in the two groups. The CD4 cell count rose similarly in the two groups during the first 12 months. However, after 12 months, patients who started cART during CHI had a better immunological response than those who resumed cART (p = 0.01); therefore, at 36 months, the gains in √CD4 cells/mm(3) and CD4% were significantly greater in patients who started treatment during CHI.
Conclusion: These results suggest that interruption of cART started during PHI has a significant, albeit modest negative impact on CD4 cell recovery on cART resumption.
Figures

References
-
- DHHS: Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. In Book Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2013.
-
- EACS . Book European AIDS Clinical Society guidelines. Europe: European AIDS Clinical Society EACS; 2013. European AIDS Clinical Society guidelines.
-
- Williams I, Churchill D, Anderson J, Boffito M, Bower M, Cairns G, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012 (Updated November 2013. All changed text is cast in yellow highlight.) HIV Med. 2014;15:1–85. doi: 10.1111/hiv.12110. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

