Cardiac mTOR rescues the detrimental effects of diet-induced obesity in the heart after ischemia-reperfusion
- PMID: 25888508
- PMCID: PMC4469881
- DOI: 10.1152/ajpheart.00008.2015
Cardiac mTOR rescues the detrimental effects of diet-induced obesity in the heart after ischemia-reperfusion
Abstract
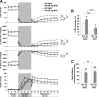
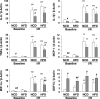
Diet-induced obesity deteriorates the recovery of cardiac function after ischemia-reperfusion (I/R) injury. While mechanistic target of rapamycin (mTOR) is a key mediator of energy metabolism, the effects of cardiac mTOR in ischemic injury under metabolic syndrome remains undefined. Using cardiac-specific transgenic mice overexpressing mTOR (mTOR-Tg mice), we studied the effect of mTOR on cardiac function in both ex vivo and in vivo models of I/R injury in high-fat diet (HFD)-induced obese mice. mTOR-Tg and wild-type (WT) mice were fed a HFD (60% fat by calories) for 12 wk. Glucose intolerance and insulin resistance induced by the HFD were comparable between WT HFD-fed and mTOR-Tg HFD-fed mice. Functional recovery after I/R in the ex vivo Langendorff perfusion model was significantly lower in HFD-fed mice than normal chow diet-fed mice. mTOR-Tg mice demonstrated better cardiac function recovery and had less of the necrotic markers creatine kinase and lactate dehydrogenase in both feeding conditions. Additionally, mTOR overexpression suppressed expression of proinflammatory cytokines, including IL-6 and TNF-α, in both feeding conditions after I/R injury. In vivo I/R models showed that at 1 wk after I/R, HFD-fed mice exhibited worse cardiac function and larger myocardial scarring along myofibers compared with normal chow diet-fed mice. In both feeding conditions, mTOR overexpression preserved cardiac function and prevented myocardial scarring. These findings suggest that cardiac mTOR overexpression is sufficient to prevent the detrimental effects of diet-induced obesity on the heart after I/R, by reducing cardiac dysfunction and myocardial scarring.
Keywords: diet-induced obesity; heart failure; mammalian target of rapamycin; metabolic syndrome; myocardial infarction; transgenic mice.
Copyright © 2015 the American Physiological Society.
Figures






References
-
- Aguilar D, Solomon SD, Kober L, Rouleau JL, Skali H, McMurray JJ, Francis GS, Henis M, O'Connor CM, Diaz R, Belenkov YN, Varshavsky S, Leimberger JD, Velazquez EJ, Califf RM, Pfeffer MA. Newly diagnosed and previously known diabetes mellitus and 1-year outcomes of acute myocardial infarction: the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation 110: 1572–1578, 2004. - PubMed
-
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120: 1640–1645, 2009. - PubMed
-
- Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol 40: 14–21, 2008. - PubMed
-
- Aoyagi T, Birumachi J, Hiroyama M, Fujiwara Y, Sanbe A, Yamauchi J, Tanoue A. Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice. Endocrinology 148: 2075–2084, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

