The surgically remediable syndrome of epilepsy associated with bottom-of-sulcus dysplasia
- PMID: 25888556
- PMCID: PMC4442099
- DOI: 10.1212/WNL.0000000000001591
The surgically remediable syndrome of epilepsy associated with bottom-of-sulcus dysplasia
Abstract
Objective: To determine clinical and EEG features that might help identify patients with epilepsy harboring small, intrinsically epileptogenic, surgically treatable, bottom-of-sulcus dysplasias (BOSDs).
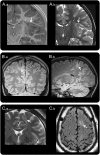
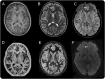
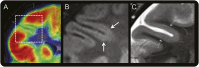
Methods: Retrospective review of clinical records, EEG, MRI, and histopathology in 32 patients with drug-resistant epilepsy and MRI-positive (72% 3.0 tesla), pathologically proven (type 2B cortical dysplasia) BOSDs operated at our centers during 2005-2013.
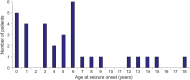
Results: Localization of BOSDs was frontal in 19, insula in 5, parietal in 5, and temporal in 3, on the convexity or interhemispheric surfaces. BOSDs were missed on initial MRI at our centers in 22% of patients. Patients presented with focal seizures during infancy in 9, preschool years in 15, and school years in 8 (median age 5 years). Seizures were stereotyped, predominantly nocturnal, and typically nonconvulsive, with semiology referable to the fronto-central or perisylvian regions. Seizures occurred at high frequency during active periods, but often went into prolonged remission with carbamazepine or phenytoin. Intellect was normal or borderline, except in patients with seizure onset during infancy. Scalp EEG frequently revealed localized interictal epileptiform discharges and ictal rhythms. Patients underwent lesionectomy (median age 14 years) guided by electrocorticography and MRI, with prior intracranial EEG monitoring in only one patient. Twenty-eight patients (88%) became seizure-free, and 20 discontinued antiepileptic medication (median follow-up 4.1 years).
Conclusions: In patients with cryptogenic focal epilepsy, this clinical presentation and course should prompt review of or repeat MRI, looking for a BOSD in the frontal, parietal, or insula cortex. If a BOSD is identified, the patient might be considered for single-stage lesionectomy.
© 2015 American Academy of Neurology.
Figures

Comment in
-
Epilepsy and the funny sulcus.Neurology. 2015 May 19;84(20):2012-3. doi: 10.1212/WNL.0000000000001603. Epub 2015 Apr 17. Neurology. 2015. PMID: 25888555 No abstract available.
References
-
- Barkovich AJ, Kuzniecky RI, Bollen AW, Grant PE. Focal transmantle dysplasia: a specific malformation of cortical development. Neurology 1997;49:1148–1152. - PubMed
-
- Mühlebner A, Coras R, Kobow K, et al. Neuropathologic measurements in focal cortical dysplasias: validation of the ILAE 2011 classification system and diagnostic implications for MRI. Acta Neuropathol 2011;123:259–272. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
