A Secreted Effector Protein of Ustilago maydis Guides Maize Leaf Cells to Form Tumors
- PMID: 25888589
- PMCID: PMC4558682
- DOI: 10.1105/tpc.114.131086
A Secreted Effector Protein of Ustilago maydis Guides Maize Leaf Cells to Form Tumors
Abstract
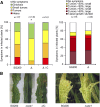
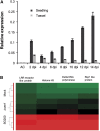
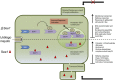
The biotrophic smut fungus Ustilago maydis infects all aerial organs of maize (Zea mays) and induces tumors in the plant tissues. U. maydis deploys many effector proteins to manipulate its host. Previously, deletion analysis demonstrated that several effectors have important functions in inducing tumor expansion specifically in maize leaves. Here, we present the functional characterization of the effector See1 (Seedling efficient effector1). See1 is required for the reactivation of plant DNA synthesis, which is crucial for tumor progression in leaf cells. By contrast, See1 does not affect tumor formation in immature tassel floral tissues, where maize cell proliferation occurs independent of fungal infection. See1 interacts with a maize homolog of SGT1 (Suppressor of G2 allele of skp1), a factor acting in cell cycle progression in yeast (Saccharomyces cerevisiae) and an important component of plant and human innate immunity. See1 interferes with the MAPK-triggered phosphorylation of maize SGT1 at a monocot-specific phosphorylation site. We propose that See1 interferes with SGT1 activity, resulting in both modulation of immune responses and reactivation of DNA synthesis in leaf cells. This identifies See1 as a fungal effector that directly and specifically contributes to the formation of leaf tumors in maize.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures






References
-
- Austin M.J., Muskett P., Kahn K., Feys B.J., Jones J.D., Parker J.E. (2002). Regulatory role of SGT1 in early R gene-mediated plant defenses. Science 295: 2077–2080. - PubMed
-
- Azevedo C., Sadanandom A., Kitagawa K., Freialdenhoven A., Shirasu K., Schulze-Lefert P. (2002). The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 295: 2073–2076. - PubMed
-
- Brefort T., Doehlemann G., Mendoza-Mendoza A., Reissmann S., Djamei A., Kahmann R. (2009). Ustilago maydis as a pathogen. Annu. Rev. Phytopathol. 47: 423–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

