Down-Regulating CsHT1, a Cucumber Pollen-Specific Hexose Transporter, Inhibits Pollen Germination, Tube Growth, and Seed Development
- PMID: 25888616
- PMCID: PMC4453785
- DOI: 10.1104/pp.15.00290
Down-Regulating CsHT1, a Cucumber Pollen-Specific Hexose Transporter, Inhibits Pollen Germination, Tube Growth, and Seed Development
Abstract
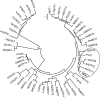
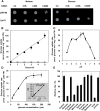
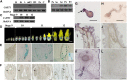
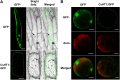
Efficient sugar transport is needed to support the high metabolic activity of pollen tubes as they grow through the pistil. Failure of transport results in male sterility. Although sucrose transporters have been shown to play a role in pollen tube development, the role of hexoses and hexose transporters is not as well established. The pollen of some species can grow in vitro on hexose as well as on sucrose, but knockouts of individual hexose transporters have not been shown to impair fertilization, possibly due to transporter redundancy. Here, the functions of CsHT1, a hexose transporter from cucumber (Cucumis sativus), are studied using a combination of heterologous expression in yeast (Saccharomyces cerevisiae), histochemical and immunohistochemical localization, and reverse genetics. The results indicate that CsHT1 is a plasma membrane-localized hexose transporter with high affinity for glucose, exclusively transcribed in pollen development and expressed both at the levels of transcription and translation during pollen grain germination and pollen tube growth. Overexpression of CsHT1 in cucumber pollen results in a higher pollen germination ratio and longer pollen tube growth than wild-type pollen in glucose- or galactose-containing medium. By contrast, antisense suppression of CsHT1 leads to inhibition of pollen germination and pollen tube elongation in the same medium and results in a decrease of seed number per fruit and seed size when antisense transgenic pollen is used to fertilize wild-type or transgenic cucumber plants. The important role of CsHT1 in pollen germination, pollen tube growth, and seed development is discussed.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures



References
-
- Bai SL, Peng YB, Cui JX, Gu HT, Xu LY, Li YQ, Xu ZH, Bai SN (2004) Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L.). Planta 220: 230–240 - PubMed
-
- Büttner M. (2010) The Arabidopsis sugar transporter (AtSTP) family: an update. Plant Biol (Stuttg) (Suppl 1) 12: 35–41 - PubMed
-
- Büttner M, Sauer N (2000) Monosaccharide transporters in plants: structure, function and physiology. Biochim Biophys Acta 1465: 263–274 - PubMed
-
- Cheung AY. (1996) The pollen tube growth pathway: its molecular and biochemical contributions and responses to pollination. Sex Plant Reprod 9: 330–336
-
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

