Characterization of HIV-1 entry inhibitors with broad activity against R5 and X4 viral strains
- PMID: 25888743
- PMCID: PMC4399250
- DOI: 10.1186/s12967-015-0461-9
Characterization of HIV-1 entry inhibitors with broad activity against R5 and X4 viral strains
Abstract
Background: Combined antiretroviral therapy has drastically reduced mortality and morbidity of HIV-infected individuals. Nevertheless long-term toxicity and appearance of viral resistance hampers the prolonged effectiveness of combination therapy, requiring a continuous input of drugs to replace those utilized in combination regimens. We here investigated the anti-HIV activity of novel derivatives of the suradista chemical class.
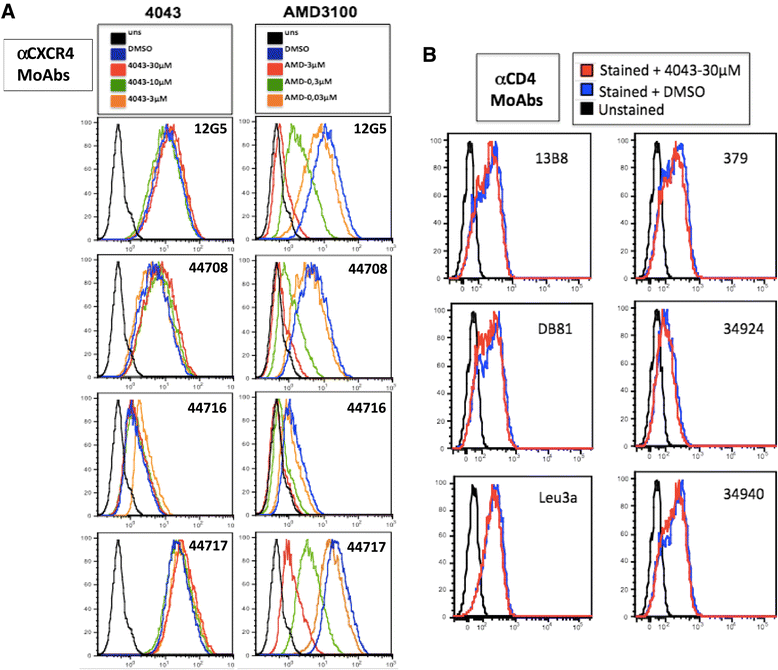
Methods: Compounds were tested on acute HIV-1 infection of activated peripheral blood mononuclear cells. HIV production was monitored by enzyme-linked immunosorbent assay measuring the protein p24 released in culture supernatants. Fusion assays were carried out to study the mechanism of action of these compounds. A modified version of a previously established recombinant vaccinia virus-based assay was used measuring activation of a reporter gene upon fusion of two distinct cell populations. Flow cytometry was performed in competition assays for the binding of several antibodies targeting different sites of the viral envelope glycoprotein gp120, or the receptor CD4, or the coreceptors CXCR4 and CCR5.
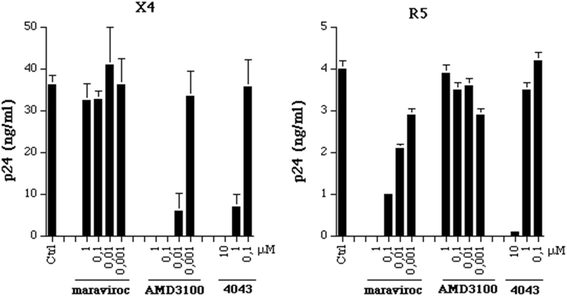
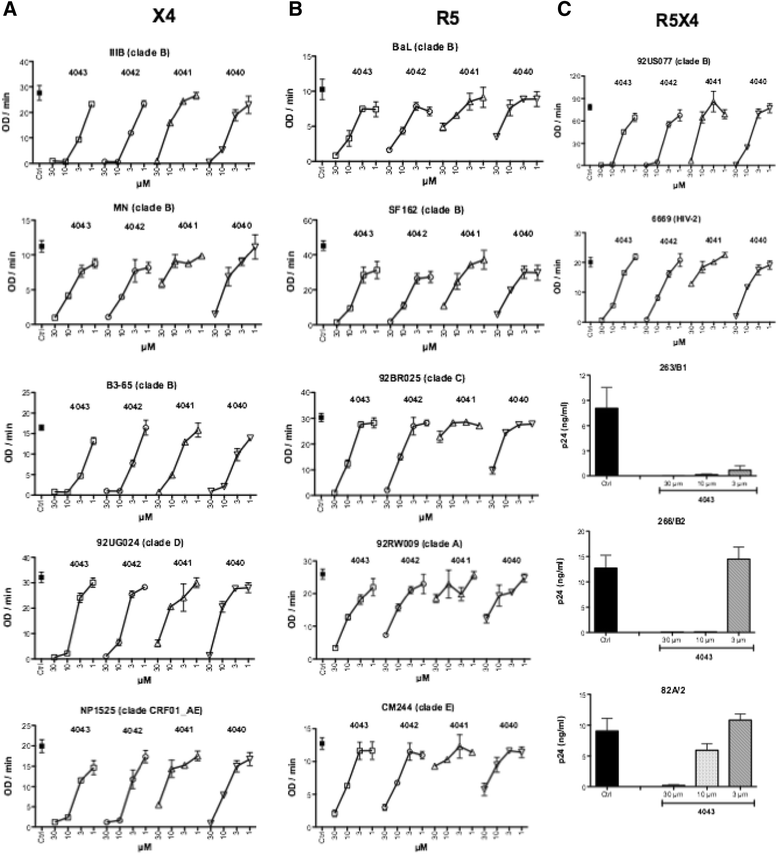
Results: Four compounds inhibited replication of a prototypic R5 (BaL) and X4 (IIIB) laboratory-adapted HIV-1 strain at low micromolar concentrations, in the absence of cytotoxicity. Approximately a ten fold greater activity was achieved against the X4 as compared to the R5 strain. The compounds blocked X4 and R5 HIV-1 fusion, a step of viral entry. This activity appeared specific for HIV-1, as entry of human herpesvirus 6 (HHV-6) and influenza virus was not substantially affected. Further investigation of the inhibitory mechanism revealed that these new molecules target the viral envelope, rather than the coreceptors, as previously shown for a congener of the same class characterized by a long plasmatic half-life. Indeed ND-4043, the most active compound, specifically competed with binding of monoclonal antibodies against the CD4-binding site (CD4-BS) and coreceptor-binding site (CoR-BS) of gp120. These compounds displayed broad anti-HIV activity, as they inhibited various primary R5, X4 and, importantly, dualtropic R5X4 HIV-1 isolates. Of the four derivatives tested, the dimeric compounds were consistently more potent than the monomeric ones.
Conclusions: Given their unique features, these molecules represent promising candidates for further development and exploitation as anti-HIV therapeutics.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

