Piscine orthoreovirus (PRV) replicates in Atlantic salmon (Salmo salar L.) erythrocytes ex vivo
- PMID: 25888832
- PMCID: PMC4350956
- DOI: 10.1186/s13567-015-0154-7
Piscine orthoreovirus (PRV) replicates in Atlantic salmon (Salmo salar L.) erythrocytes ex vivo
Abstract
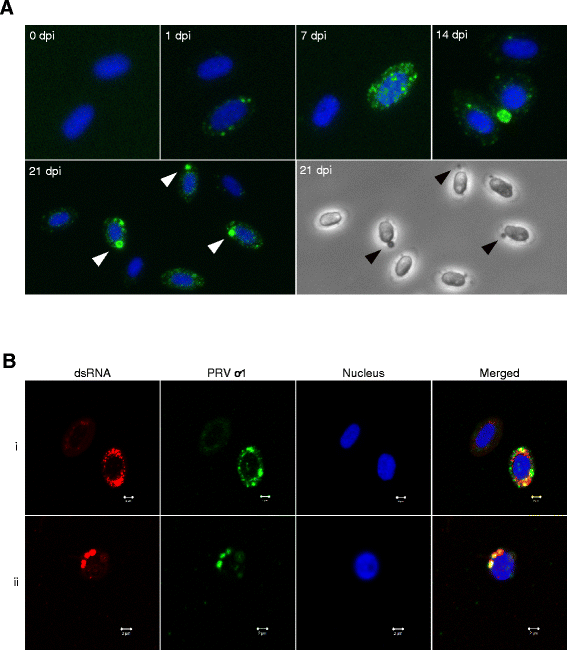
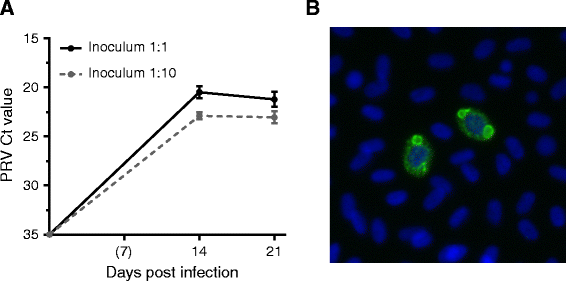
Piscine orthoreovirus (PRV) is a reovirus that has predominantly been detected in Atlantic salmon (Salmo salar L.). PRV is associated with heart and skeletal muscle inflammation (HSMI) in farmed Atlantic salmon, and recently erythrocytes were identified as major target cells. The study of PRV replication and pathogenesis of the infection has been impeded by the inability to propagate PRV in vitro. In this study we developed an ex vivo cultivation system for PRV in Atlantic salmon erythrocytes. PRV was successfully passaged to naïve erythrocytes using lysates of blood cells from infected salmon. During cultivation a significant increase in viral load was observed by RT-qPCR and flow cytometry, which coincided with the formation of cytoplasmic inclusions. The inclusions resembled viral factories and contained both PRV protein and dsRNA. In addition, the erythrocytes generated an antiviral immune gene activation after PRV infection, with significant up-regulation of IFN-α, RIG-I, Mx and PKR transcripts. Supernatants from the first passage successfully transmitted virus to naïve erythrocytes. This study demonstrates that PRV replicates in Atlantic salmon erythrocytes ex vivo. The ex vivo infection model closely reflects the situation in vivo and can be used to study the infection and replication mechanisms of PRV, as well as the antiviral immune responses of salmonid erythrocytes.
Figures



References
-
- Palacios G, Løvoll M, Tengs T, Hornig M, Hutchison S, Hui J, Kongtorp RT, Savji N, Bussetti AV, Solovyov A, Kristoffersen AB, Celone C, Street C, Trifonov V, Hirschberg DL, Rabadan R, Egholm M, Rimstad E, Lipkin WI. Heart and skeletal muscle inflammation of farmed salmon is associated with infection with a novel reovirus. PLoS One. 2010;5:e11487. doi: 10.1371/journal.pone.0011487. - DOI - PMC - PubMed
-
- Kibenge MJ, Iwamoto T, Wang Y, Morton A, Godoy MG, Kibenge FS. Whole-genome analysis of piscine reovirus (PRV) shows PRV represents a new genus in family Reoviridae and its genome segment S1 sequences group it into two separate sub-genotypes. Virol J. 2013;10:230. doi: 10.1186/1743-422X-10-230. - DOI - PMC - PubMed
-
- Marty GD, Morrison DB, Bidulka J, Joseph T, Siah A. Piscine reovirus in wild and farmed salmonids in British Columbia, Canada: 1974–2013. J Fish Dis, in press. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

