Metastatic neuroblastoma cancer stem cells exhibit flexible plasticity and adaptive stemness signaling
- PMID: 25888913
- PMCID: PMC4396071
- DOI: 10.1186/s13287-015-0002-8
Metastatic neuroblastoma cancer stem cells exhibit flexible plasticity and adaptive stemness signaling
Erratum in
-
Erratum to: Metastatic neuroblastoma cancer stem cells exhibit flexible plasticity and adaptive stemness signaling.Stem Cell Res Ther. 2016 Feb 18;7:32. doi: 10.1186/s13287-016-0291-6. Stem Cell Res Ther. 2016. PMID: 26892617 Free PMC article. No abstract available.
Abstract
Introduction: High-risk neuroblastoma (HR-NB) presenting with hematogenous metastasis is one of the most difficult cancers to cure. Patient survival is poor. Aggressive tumors contain populations of rapidly proliferating clonogens that exhibit stem cell properties, cancer stem cells (CSCs). Conceptually, CSCs that evade intensive multimodal therapy dictate tumor progression, relapse/recurrence, and poor clinical outcomes. Herein, we investigated the plasticity and stem-cell related molecular response of aggressive metastatic neuroblastoma cells that fit the CSC model.
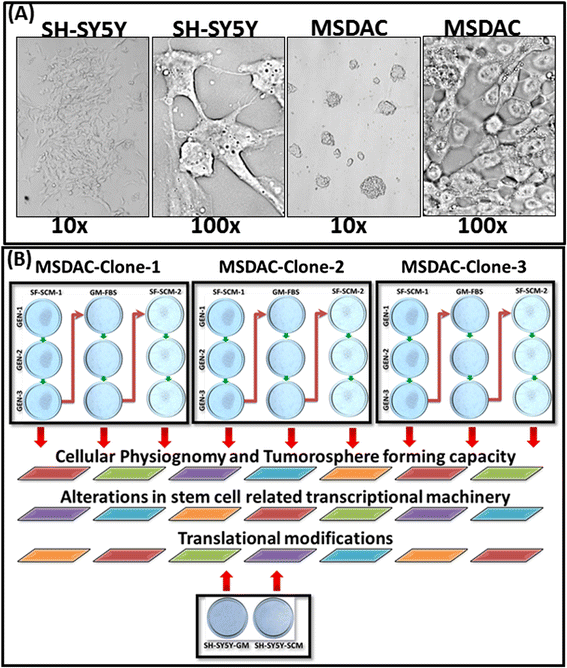
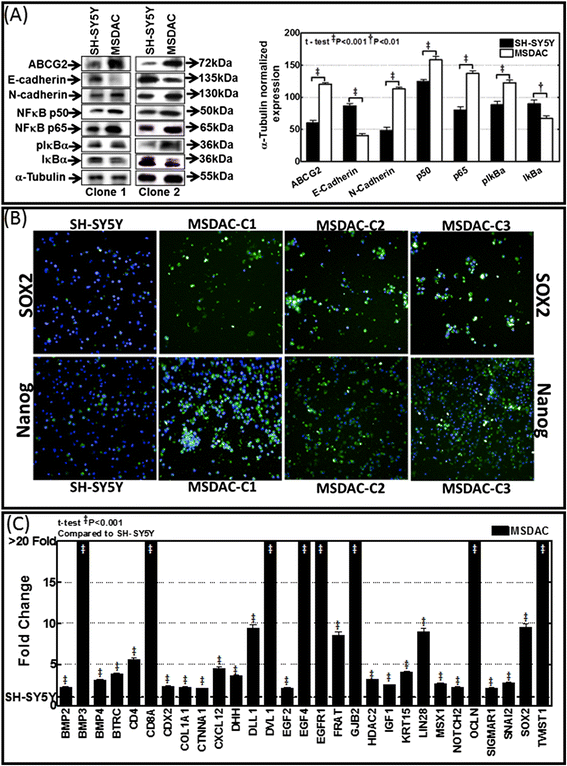
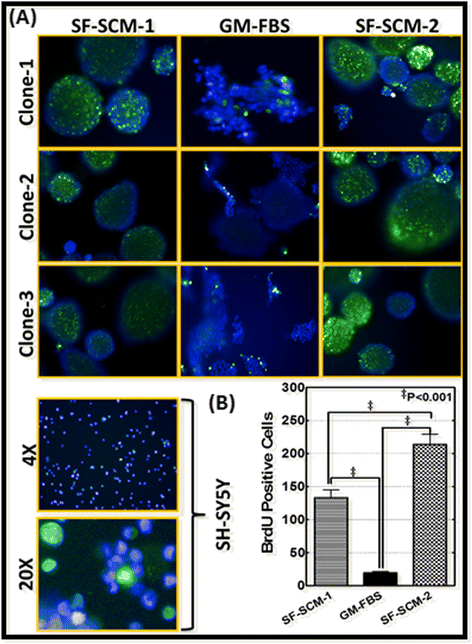
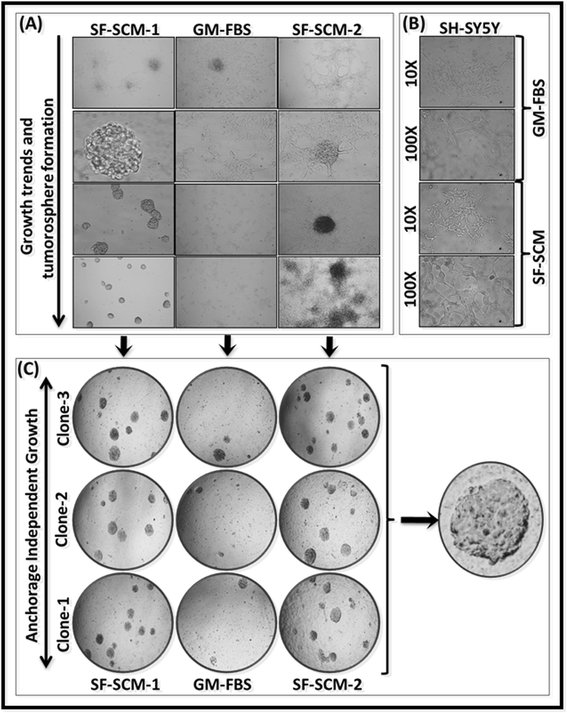
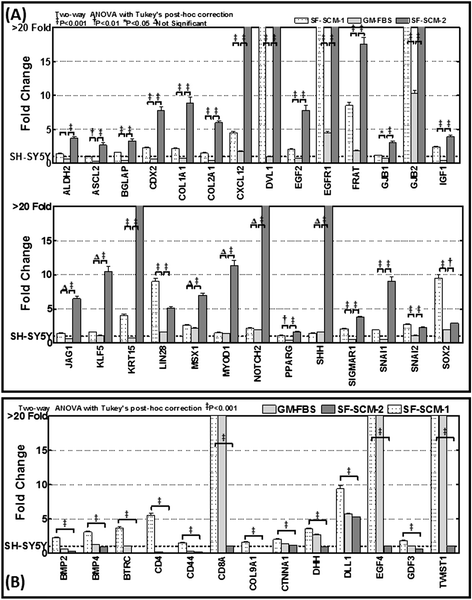
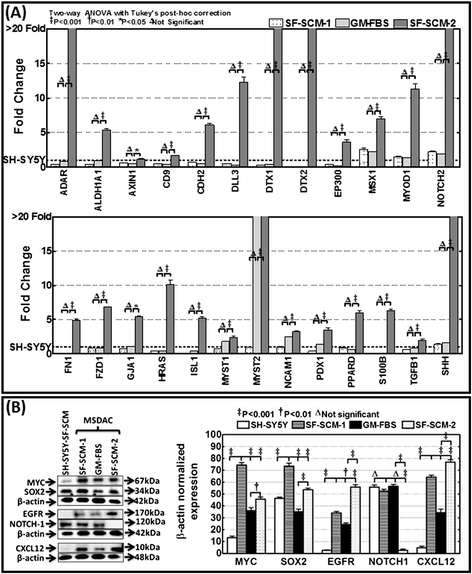
Methods: Well-characterized clones of metastatic site-derived aggressive cells (MSDACs) from a manifold of metastatic tumors of clinically translatable HR-NB were characterized for their CSC fit by examining epithelial-to-mesenchymal transition (EMT) (E-cadherin, N-Cadherin), survival (NFκB P65, p50, IκB and pIκB) and drug resistance (ABCG2) by immunoblotting; pluripotency maintenance (Nanog, SOX2) by immunofluorescence; and EMT and stemness related transcription of 93 genes by QPCR profiling. Plasticity of MSDACs under sequential alternation of culture conditions with serum and serum-free stem-cell conditions was assessed by clonal expansion (BrdU incorporation), tumorosphere formation (anchorage independent growth), EMT and stemness related transcriptome (QPCR profiling) and validated with MYC, SOX2, EGFR, NOTCH1 and CXCL2 immunoblotting.
Results: HR-NB MSDACs maintained in alternated culture conditions, serum-free stem cell medium to growth medium with serum and vice versa identified its flexible revocable plasticity characteristics. We observed signatures of stem cell-related molecular responses consistent with phenotypic conversions. Successive reintroduction to the favorable niche not only regained identical EMT, self-renewal capacity, pluripotency maintenance, and other stem cell-related signaling events, but also instigated additional events depicting aggressive adaptive plasticity.
Conclusions: Together, these results demonstrated the flexible plasticity of HR-NB MSDACs that typically fit the CSC model, and further identified the intrinsic adaptiveness of the successive phenotype switching that clarifies the heterogeneity of HR-NB. Moreover, the continuous ongoing acquisition of stem cell-related molecular rearrangements may hold the key to the switch from favorable disease to HR-NB.
Figures
Similar articles
-
Acquired genetic alterations in tumor cells dictate the development of high-risk neuroblastoma and clinical outcomes.BMC Cancer. 2015 Jul 10;15:514. doi: 10.1186/s12885-015-1463-y. BMC Cancer. 2015. PMID: 26159519 Free PMC article.
-
Different Effects of BORIS/CTCFL on Stemness Gene Expression, Sphere Formation and Cell Survival in Epithelial Cancer Stem Cells.PLoS One. 2015 Jul 17;10(7):e0132977. doi: 10.1371/journal.pone.0132977. eCollection 2015. PLoS One. 2015. PMID: 26185996 Free PMC article.
-
NANOGP8 is the key regulator of stemness, EMT, Wnt pathway, chemoresistance, and other malignant phenotypes in gastric cancer cells.PLoS One. 2018 Apr 24;13(4):e0192436. doi: 10.1371/journal.pone.0192436. eCollection 2018. PLoS One. 2018. PMID: 29689047 Free PMC article.
-
Cancer stem cell (a)symmetry & plasticity: Tumorigenesis and therapy relevance.Life Sci. 2019 Aug 15;231:116520. doi: 10.1016/j.lfs.2019.05.076. Epub 2019 May 31. Life Sci. 2019. PMID: 31158379 Review.
-
The Role of Cancer Stem Cells in Recurrent and Drug-Resistant Lung Cancer.Adv Exp Med Biol. 2016;890:57-74. doi: 10.1007/978-3-319-24932-2_4. Adv Exp Med Biol. 2016. PMID: 26703799 Review.
Cited by
-
Prognostic significance of NT5E/CD73 in neuroblastoma and its function in CSC stemness maintenance.Cell Biol Toxicol. 2023 Jun;39(3):967-989. doi: 10.1007/s10565-021-09658-1. Epub 2021 Nov 13. Cell Biol Toxicol. 2023. PMID: 34773529
-
Serum-circulating miRNAs predict neuroblastoma progression in mouse model of high-risk metastatic disease.Oncotarget. 2016 Apr 5;7(14):18605-19. doi: 10.18632/oncotarget.7615. Oncotarget. 2016. PMID: 26921195 Free PMC article.
-
Cancer Stem Cells and Neuroblastoma: Characteristics and Therapeutic Targeting Options.Front Endocrinol (Lausanne). 2019 Nov 19;10:782. doi: 10.3389/fendo.2019.00782. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31803140 Free PMC article. Review.
-
Acquired genetic alterations in tumor cells dictate the development of high-risk neuroblastoma and clinical outcomes.BMC Cancer. 2015 Jul 10;15:514. doi: 10.1186/s12885-015-1463-y. BMC Cancer. 2015. PMID: 26159519 Free PMC article.
-
Neuroblastoma Origin and Therapeutic Targets for Immunotherapy.J Immunol Res. 2018 Jul 11;2018:7394268. doi: 10.1155/2018/7394268. eCollection 2018. J Immunol Res. 2018. PMID: 30116755 Free PMC article. Review.
References
-
- Marc TG, Gurney JG, Smith MA, Olshan AF. Sympathetic nervous system tumors. In: Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute; 1999. p. 65–72. NIH Pub. No. 99-4649(ICCC IV).
-
- Gurney JG, Smith MA, Ross JA. Cancer among infants. In: Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute; 1999. p. 149–56. NIH Pub. No. 99–4649 (XII).
-
- Shimada H, Chatten J, Newton WA, Jr, Sachs N, Hamoudi AB, Chiba T, et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984;73:405–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

