Interaction between mesenchymal stem cells and endothelial cells restores endothelial permeability via paracrine hepatocyte growth factor in vitro
- PMID: 25888925
- PMCID: PMC4431320
- DOI: 10.1186/s13287-015-0025-1
Interaction between mesenchymal stem cells and endothelial cells restores endothelial permeability via paracrine hepatocyte growth factor in vitro
Abstract
Introduction: Mesenchymal stem cells (MSCs) have potent stabilising effects on vascular endothelium injury, inhibiting endothelial permeability in lung injury via paracrine hepatocyte growth factor (HGF). Recently, it has been indicated that MSCs secrete more factors by MSC-endothelial cell (MSC-EC) interactions. We hypothesised that MSC-EC interactions restore endothelial permeability induced by lipopolysaccharide (LPS) via paracrine HGF.
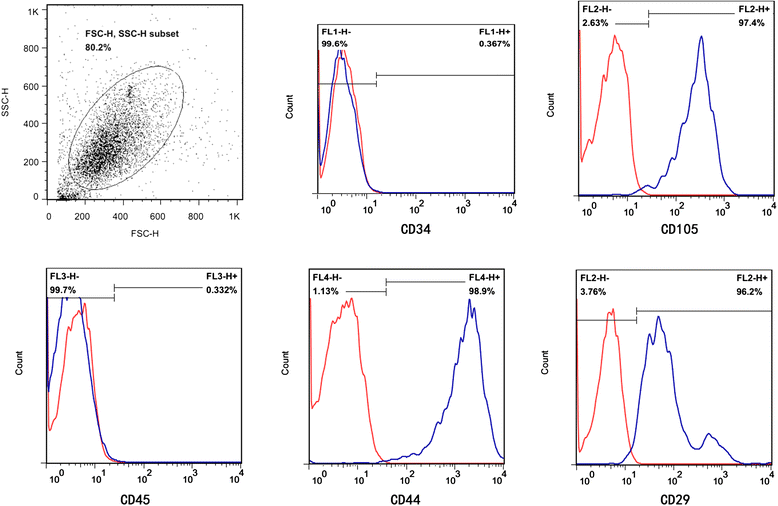
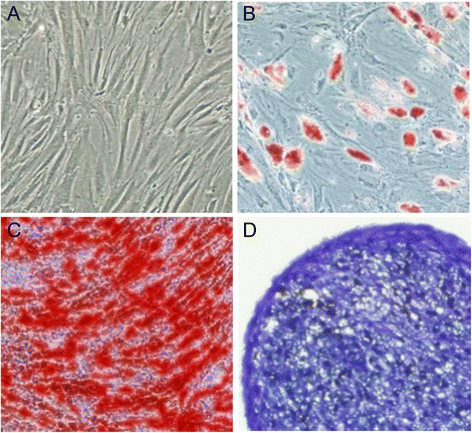
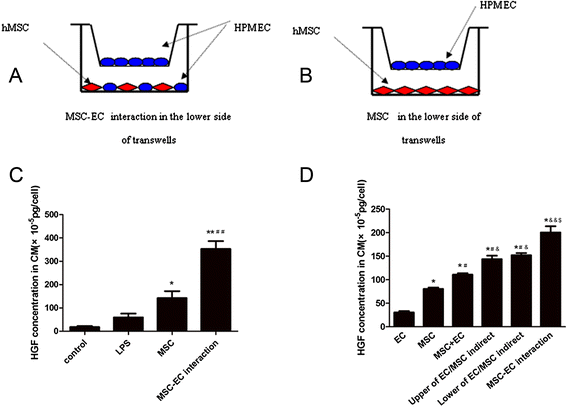
Methods: We investigated the endothelial permeability induced by LPS under two co-culture conditions. Human pulmonary microvascular endothelial cells (HPMECs) were added into the upper chambers of cell-culture inserts, while two different co-culture conditions were used in the lower side of the transwells, as follows: (1) MSC-EC interaction group: MSCs and HPMECs contact co-culture; (2) MSC group: MSCs only. The endothelial paracellular and transcellular permeabilities in the upper side of transwells were detected. Then the concentration of HGF was measured in the culture medium by using an enzyme-linked immunosorbent assay kit, followed by neutralisation of HGF with anti-HGF antibody in the co-culture medium. In addition, adherens junction and cytoskeleton protein expressions were measured by Western blot and immunofluorescence. HPMEC proliferation was analysed by bromodeoxyuridine incorporation assay.
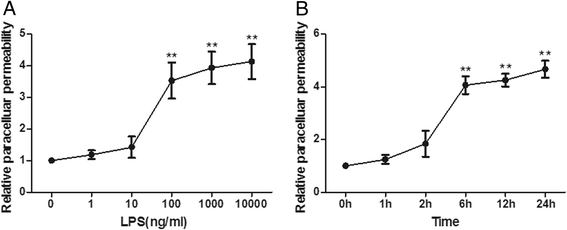
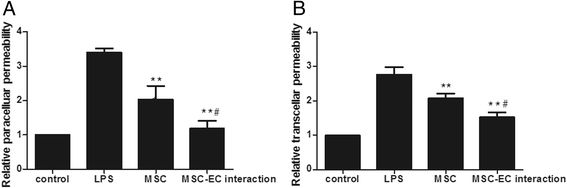
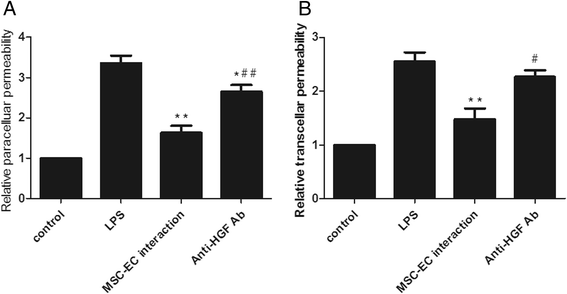
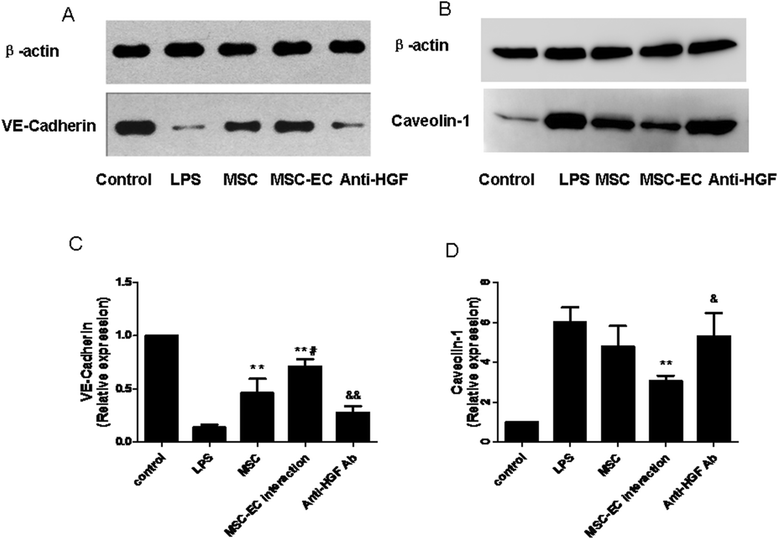
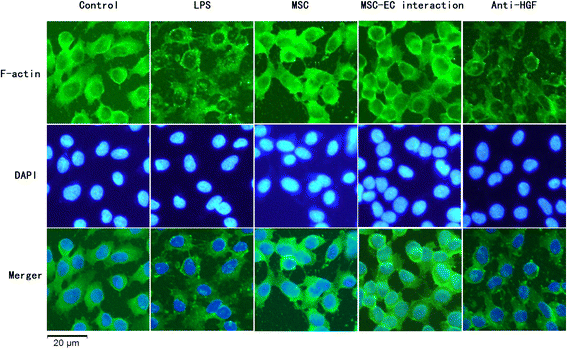
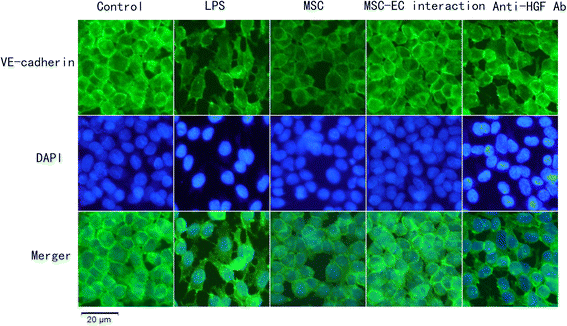
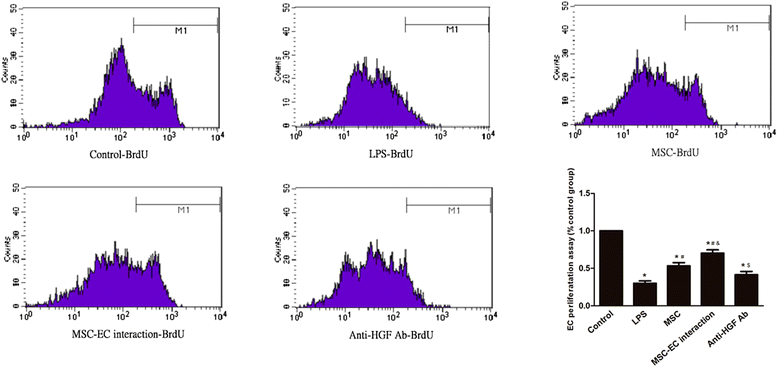
Results: The paracellular permeability significantly increased after LPS stimulation in a dose-dependent and time-dependent manner. Meanwhile, MSC-EC interaction more significantly decreased endothelial paracellular and transcellular permeability induced by LPS. Moreover, HGF levels in the MSC-EC interaction group were much higher than those of the MSC group. However, neutralising HGF with anti-HGF antibody inhibited the role of MSC-EC interaction in improving endothelial permeability. Compared with the MSC group, MSC-EC interaction increased vascular endothelial (VE)-cadherin and occludin protein expression, reduced caveolin-1 protein expression in HPMECs, and restored remodelling of F-actin and junctional localisation of VE-cadherin. Furthermore, the proliferation ratio in the MSC-EC interaction group was higher than that of the MSC group. However, the effects of MSCs were significantly blocked by anti-HGF antibody.
Conclusions: These data suggested that MSC-EC interaction decreased endothelial permeability induced by LPS, which was attributed mainly to HGF secreted by MSCs. The main mechanisms by which HGF restored the integrity of endothelial monolayers were remodelling of endothelial intercellular junctions, decreasing caveolin-1 protein expression, and inducing proliferation in HPMECs.
Figures
Similar articles
-
Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway.Stem Cell Res Ther. 2015 Dec 16;6:250. doi: 10.1186/s13287-015-0257-0. Stem Cell Res Ther. 2015. PMID: 26674641 Free PMC article.
-
Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF).Stem Cell Res Ther. 2017 Sep 29;8(1):211. doi: 10.1186/s13287-017-0662-7. Stem Cell Res Ther. 2017. PMID: 28969681 Free PMC article.
-
[The role and mechanism of mesenchymal stem cell in modulating human pulmonary microvascule endothelial cell permeability via paracrine hepatocyte growth factor].Zhonghua Jie He He Hu Xi Za Zhi. 2017 Nov 12;40(11):855-858. doi: 10.3760/cma.j.issn.1001-0939.2017.11.011. Zhonghua Jie He He Hu Xi Za Zhi. 2017. PMID: 29320834 Chinese.
-
Recent advances in the therapeutic efficacy of hepatocyte growth factor gene-modified mesenchymal stem cells in multiple disease settings.J Cell Mol Med. 2022 Sep;26(18):4745-4755. doi: 10.1111/jcmm.17497. Epub 2022 Aug 3. J Cell Mol Med. 2022. PMID: 35922965 Free PMC article. Review.
-
Potential role of a novel vascular modulator, hepatocyte growth factor (HGF), in cardiovascular disease: characterization and regulation of local HGF system.J Atheroscler Thromb. 1997;4(1):12-9. doi: 10.5551/jat1994.4.12. J Atheroscler Thromb. 1997. PMID: 9583349 Review.
Cited by
-
More than Antibiotics: Latest Therapeutics in the Treatment and Prevention of Ocular Surface Infections.J Clin Med. 2022 Jul 19;11(14):4195. doi: 10.3390/jcm11144195. J Clin Med. 2022. PMID: 35887958 Free PMC article. Review.
-
Biomimetic scaffold-based stem cell transplantation promotes lung regeneration.Bioeng Transl Med. 2023 May 5;8(4):e10535. doi: 10.1002/btm2.10535. eCollection 2023 Jul. Bioeng Transl Med. 2023. PMID: 37476061 Free PMC article.
-
Human Multilineage-differentiating Stress-Enduring Cells Exert Pleiotropic Effects to Ameliorate Acute Lung Ischemia-Reperfusion Injury in a Rat Model.Cell Transplant. 2018 Jun;27(6):979-993. doi: 10.1177/0963689718761657. Epub 2018 Apr 30. Cell Transplant. 2018. PMID: 29707971 Free PMC article.
-
Advances in mesenchymal stromal cell therapy for acute lung injury/acute respiratory distress syndrome.Front Cell Dev Biol. 2022 Aug 10;10:951764. doi: 10.3389/fcell.2022.951764. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36036014 Free PMC article. Review.
-
Endothelial cells from pulmonary endarterectomy specimens possess a high angiogenic potential and express high levels of hepatocyte growth factor.BMC Pulm Med. 2018 Dec 29;18(1):197. doi: 10.1186/s12890-018-0769-3. BMC Pulm Med. 2018. PMID: 30594174 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

