Biodegradable nanoparticles sequentially decorated with Polyethyleneimine and Hyaluronan for the targeted delivery of docetaxel to airway cancer cells
- PMID: 25888948
- PMCID: PMC4424546
- DOI: 10.1186/s12951-015-0088-2
Biodegradable nanoparticles sequentially decorated with Polyethyleneimine and Hyaluronan for the targeted delivery of docetaxel to airway cancer cells
Abstract
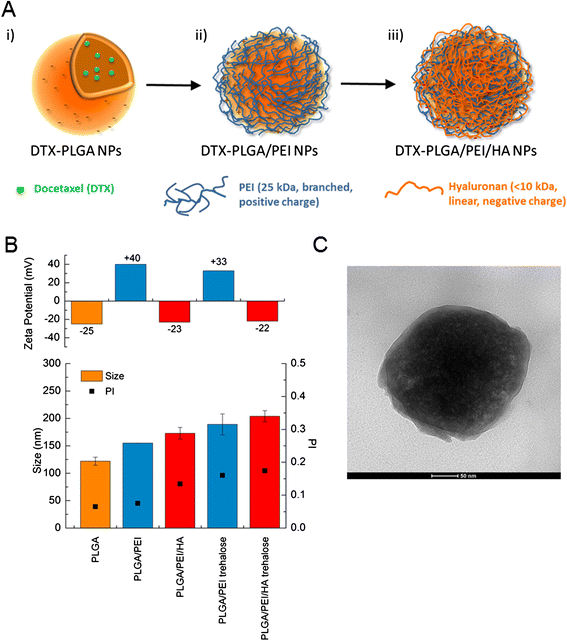
Background: Novel polymeric nanoparticles (NPs) specifically designed for delivering chemotherapeutics in the body and aimed at improving treatment activity and selectivity, cover a very relevant area in the field of nanomedicine. Here, we describe how to build a polymer shell of Hyaluronan (HA) and Polyethyleneimine (PEI) on biodegradable NPs of poly(lactic-co-glycolic) acid (PLGA) through electrostatic interactions and to achieve NPs with unique features of sustained delivery of a docetaxel (DTX) drug cargo as well as improved intracellular uptake.
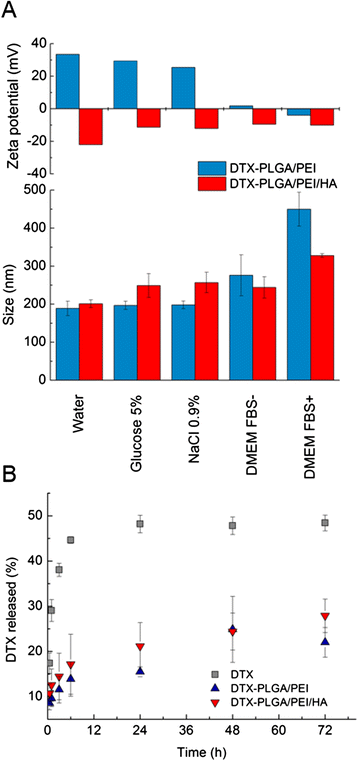
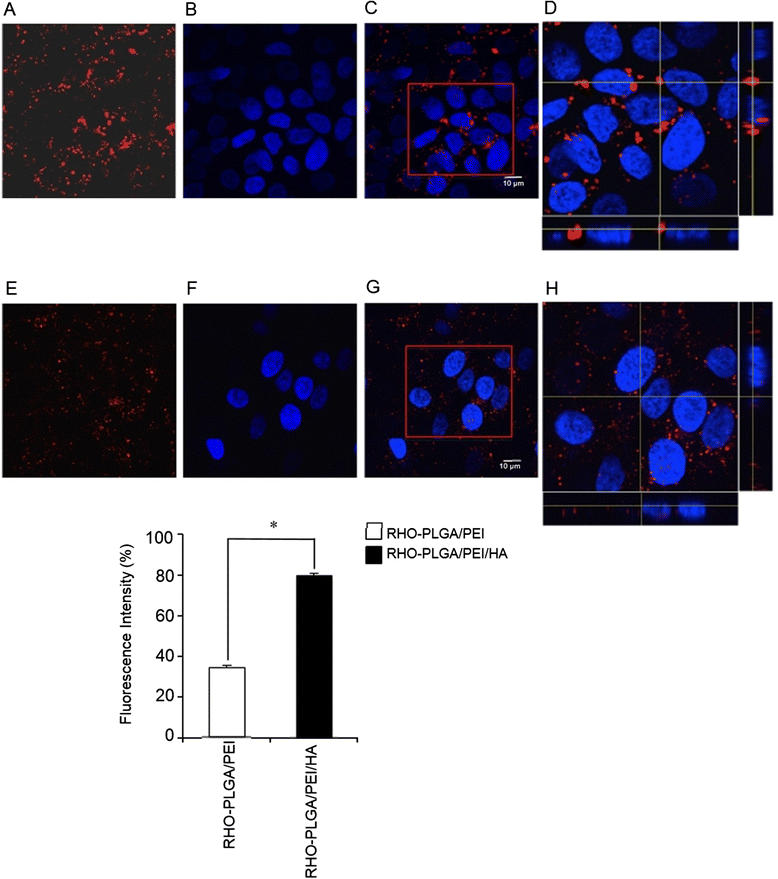
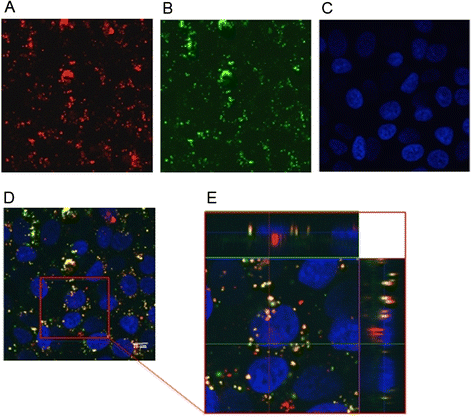
Results: A stable PEI or HA/PEI shell could be obtained by careful selection of layering conditions. NPs with exquisite stability in salt and protein-rich media, with size and surface charge matching biological requirements for intravenous injection and endowed with sustained DTX release could be obtained. Cytotoxicity, uptake and activity of both PLGA/PEI/HA and PLGA/PEI NPs were evaluated in CD44(+) (A549) and CD44(-) (Calu-3) lung cancer cells. In fact, PEI-coated NPs can be formed after degradation/dissociation of the surface HA because of the excess hyaluronidases overexpressed in tumour interstitium. There was no statistically significant cytotoxic effect of PLGA/PEI/HA and PLGA/PEI NPs in both cell lines, thus suggesting that introduction of PEI in NP shell was not hampered by its intrinsic toxicity. Intracellular trafficking of NPs fluorescently labeled with Rhodamine (RHO) (RHO-PLGA/PEI/HA and RHO-PLGA/PEI NPs) demonstrated an increased time-dependent uptake only for RHO-PLGA/PEI/HA NPs in A549 cells as compared to Calu-3 cells. As expected, RHO-PLGA/PEI NP uptake in A549 cells was comparable to that observed in Calu-3 cells. RHO-PLGA/PEI/HA NPs internalized into A549 cells showed a preferential perinuclear localization. Cytotoxicity data in A549 cells suggested that DTX delivered through PLGA/PEI/HA NPs exerted a more potent antiproliferative activity than free DTX. Furthermore, DTX-PLGA/PEI NPs, as hypothetical result of hyaluronidase-mediated degradation in tumor interstitium, were still able to improve the cytotoxic activity of free DTX.
Conclusions: Taken together, results lead us to hypothesize that biodegradable NPs coated with a PEI/HA shell represent a very promising system to treat CD44 overexpressing lung cancer. In principle, this novel nanocarrier can be extended to different single drugs and drug combinations taking advantage of the shell and core properties.
Figures



Similar articles
-
Hyaluronic acid-decorated poly(lactic-co-glycolic acid) nanoparticles for combined delivery of docetaxel and tanespimycin.Carbohydr Polym. 2015 Jun 5;123:313-23. doi: 10.1016/j.carbpol.2015.01.064. Epub 2015 Feb 7. Carbohydr Polym. 2015. PMID: 25843864
-
Hyaluronic acid coated PLGA nanoparticulate docetaxel effectively targets and suppresses orthotopic human lung cancer.J Control Release. 2017 Aug 10;259:76-82. doi: 10.1016/j.jconrel.2016.12.024. Epub 2016 Dec 24. J Control Release. 2017. PMID: 28027947
-
Development and mechanistic insight into enhanced cytotoxic potential of hyaluronic acid conjugated nanoparticles in CD44 overexpressing cancer cells.Eur J Pharm Sci. 2017 Jan 15;97:79-91. doi: 10.1016/j.ejps.2016.10.028. Epub 2016 Oct 29. Eur J Pharm Sci. 2017. PMID: 27989859
-
PLGA-based nanoparticles as cancer drug delivery systems.Asian Pac J Cancer Prev. 2014;15(2):517-35. doi: 10.7314/apjcp.2014.15.2.517. Asian Pac J Cancer Prev. 2014. PMID: 24568455 Review.
-
Biomedical applications of PLGA nanoparticles in nanomedicine: advances in drug delivery systems and cancer therapy.Expert Opin Drug Deliv. 2023 Jul-Dec;20(7):937-954. doi: 10.1080/17425247.2023.2223941. Epub 2023 Jun 23. Expert Opin Drug Deliv. 2023. PMID: 37294853 Review.
Cited by
-
Anti-α4β7 monoclonal antibody-conjugated nanoparticles block integrin α4β7 on intravaginal T cells in rhesus macaques.Sci Adv. 2020 Aug 21;6(34):eabb9853. doi: 10.1126/sciadv.abb9853. Print 2020 Aug. Sci Adv. 2020. PMID: 32937372 Free PMC article.
-
Polymerized porin as a novel delivery platform for coronavirus vaccine.J Nanobiotechnology. 2022 Jun 7;20(1):260. doi: 10.1186/s12951-022-01469-8. J Nanobiotechnology. 2022. PMID: 35672856 Free PMC article.
-
Enhancement of 5-FU sensitivity by the proapoptotic rpL3 gene in p53 null colon cancer cells through combined polymer nanoparticles.Oncotarget. 2016 Nov 29;7(48):79670-79687. doi: 10.18632/oncotarget.13216. Oncotarget. 2016. PMID: 27835895 Free PMC article.
-
Therapeutic Approaches Targeting Nucleolus in Cancer.Cells. 2019 Sep 16;8(9):1090. doi: 10.3390/cells8091090. Cells. 2019. PMID: 31527430 Free PMC article. Review.
-
STAT3/NF-κB signalling disruption in M2 tumour-associated macrophages is a major target of PLGA nanocarriers/PD-L1 antibody immunomodulatory therapy in breast cancer.Br J Pharmacol. 2021 Jun;178(11):2284-2304. doi: 10.1111/bph.15373. Epub 2021 Mar 31. Br J Pharmacol. 2021. PMID: 33434950 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

