Comparative analysis of cytokine transcript profiles within mediastinal lymph node compartments of pigs after infection with porcine reproductive and respiratory syndrome genotype 1 strains differing in pathogenicity
- PMID: 25889072
- PMCID: PMC4364558
- DOI: 10.1186/s13567-015-0161-8
Comparative analysis of cytokine transcript profiles within mediastinal lymph node compartments of pigs after infection with porcine reproductive and respiratory syndrome genotype 1 strains differing in pathogenicity
Abstract
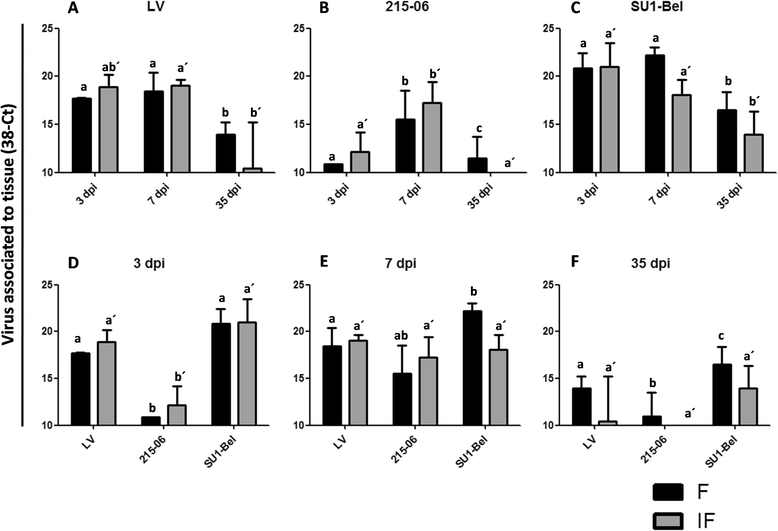
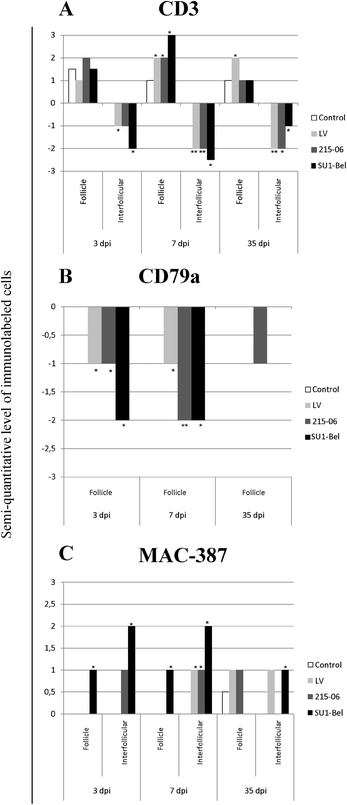
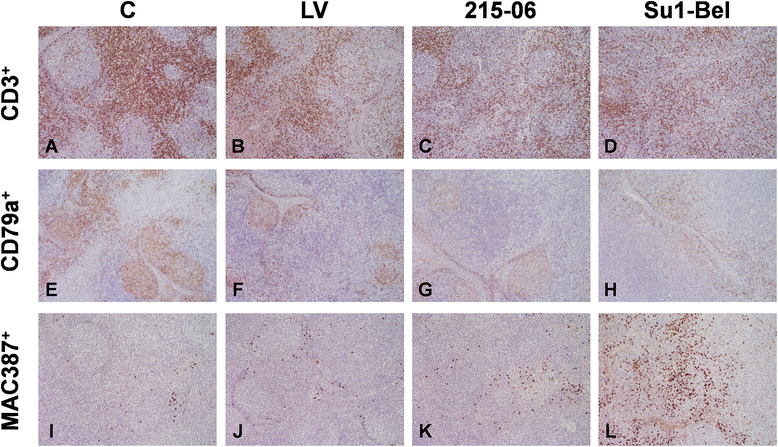
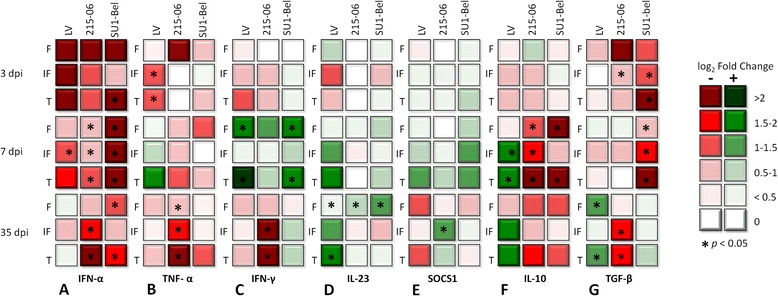
Porcine reproductive and respiratory syndrome virus (PRRSV) induces a weak immune response enabling it to persist in different organs of infected pigs. This has been attributed to the ability of PRRSV to influence the induction of cytokine responses. In this study, we investigated the cytokine transcriptional profiles in different compartments of the mediastinal lymph node of pigs infected with three genotype 1 PRRSV strains of differing pathogenicity: the low virulence prototype Lelystad virus (LV), and UK field strain 215-06 and the highly virulent subtype 3 SU1-Bel isolate from Belarus. We have used a combination of laser capture micro-dissection (LCM) followed by real time quantitative PCR (RT-qPCR) and immunohistochemical (IHC) detection of immune cell markers (CD3, CD79a and MAC387) and RT-qPCR quantification of PRRSV and cytokine transcripts. Compared to mock infected pigs, we found a significant downregulation of TNF-α and IFN-α in follicular and interfollicular areas of the mediastinal lymph node from 3 days post-infection (dpi) in animals infected with all three strains. This was accompanied by a transient B cell depletion and T cell and macrophage infiltration in the follicles together with T cell depletion in the interfollicular areas. A delayed upregulation of IFN-γ and IL-23p19 was observed mainly in the follicles. The PRRSV load was higher in all areas and time-points studied in the animals infected with the SU1-Bel strain. This paper describes the first application of LCM to study the cytokine transcript profiles and virus distribution in different compartments of the lymph node of pigs.
Figures
References
-
- Zimmerman JJ, Benfield DA, Murtaugh MP, Osorio F, Stenvenson GW, Torremorell M (2006) Porcine reproductive and respiratory syndrome virus (porcine arterivirus). InStraw BE, Zimmerman JJ, D’Allaire S, Taylor DJ (eds) Ames, Iowa5. Holtkamp DJ, Kliebenstein JB, Neumann EJ, Zimmerman JJ, Rotto HF, Yoder TK, Wang C, Yeske PE, Mowrer CL, Haley CA (2013) Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Prod 21:72–84
-
- Holtkamp DJ, Kliebenstein JB, Neumann EJ, Zimmerman JJ, Rotto HF, Yoder TK, Wang C, Yeske PE, Mowrer CL, Haley CA. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Prod. 2013;21:72–84.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

