SapC-DOPS nanovesicles induce Smac- and Bax-dependent apoptosis through mitochondrial activation in neuroblastomas
- PMID: 25889084
- PMCID: PMC4397704
- DOI: 10.1186/s12943-015-0336-y
SapC-DOPS nanovesicles induce Smac- and Bax-dependent apoptosis through mitochondrial activation in neuroblastomas
Abstract
Background: High toxicity, morbidity and secondary malignancy render chemotherapy of neuroblastoma inefficient, prompting the search for novel compounds. Nanovesicles offer great promise in imaging and treatment of cancer. SapC-DOPS, a stable nanovesicle formed from the lysosomal protein saposin C and dioleoylphosphatidylserine possess strong affinity for abundantly exposed surface phosphatidylserine on cancer cells. Here, we show that SapC-DOPS effectively targets and suppresses neuroblastoma growth and elucidate the molecular mechanism of SapC-DOPS action in neuroblastoma in vitro.
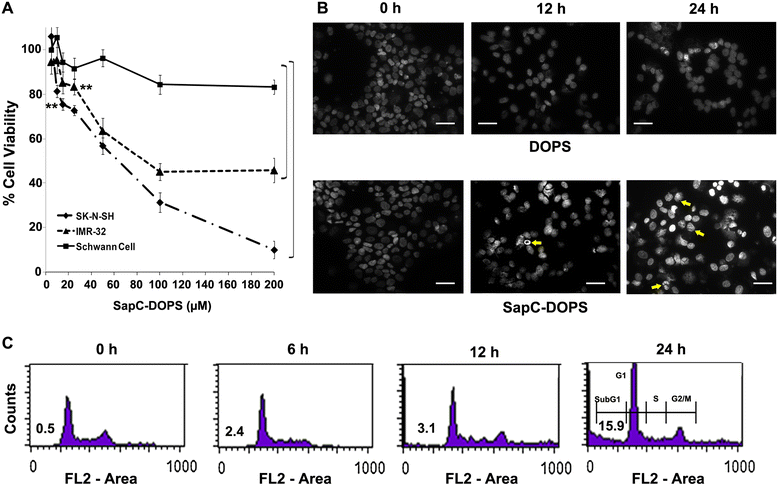
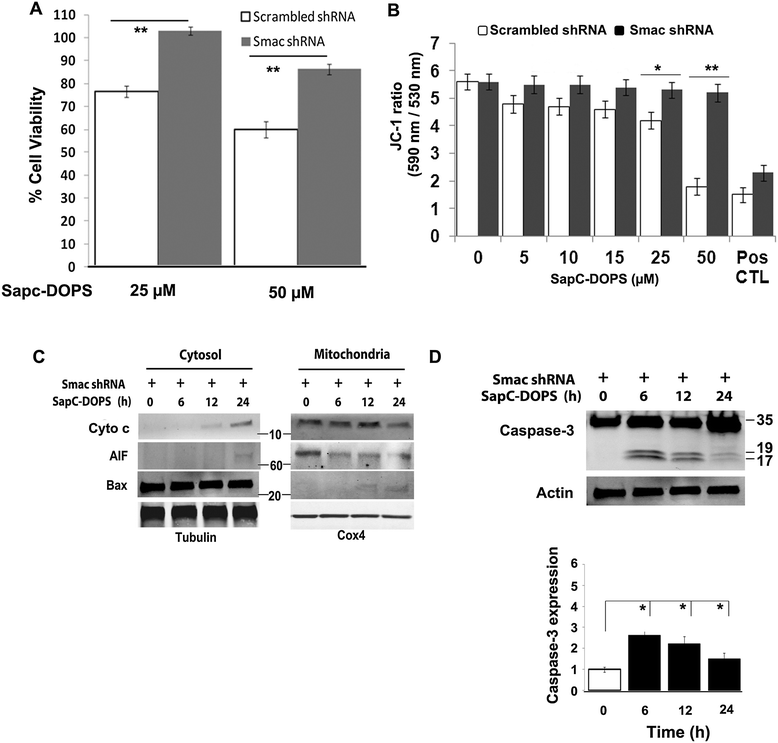
Methods: In vivo targeting of neuroblastoma was assessed in xenograft mice injected intravenously with fluorescently-labeled SapC-DOPS. Xenografted tumors were also used to demonstrate its therapeutic efficacy. Apoptosis induction in vivo was evaluated in tumor sections using the TUNEL assay. The mechanisms underlying the induction of apoptosis by SapC-DOPS were addressed through measurements of cell viability, mitochondrial membrane potential (ΔΨM), flow cytometric DNA fragmentation assays and by immunoblot analysis of second mitochondria-derived activator of caspases (Smac), Bax, Cytochrome c (Cyto c) and Caspase-3 in the cytosol or in mitochondrial fractions of cultured neuroblastoma cells.
Results: SapC-DOPS showed specific targeting and prevented the growth of human neuroblastoma xenografts in mice. In neuroblastoma cells in vitro, apoptosis occurred via a series of steps that included: (1) loss of ΔΨM and increased mitochondrial superoxide formation; (2) cytosolic release of Smac, Cyto c, AIF; and (3) mitochondrial translocation and polymerization of Bax. ShRNA-mediated Smac knockdown and V5 peptide-mediated Bax inhibition decreased cytosolic Smac and Cyto c release along with caspase activation and abrogated apoptosis, indicating that Smac and Bax are critical mediators of SapC-DOPS action. Similarly, pretreatment with the mitochondria-stabilizing agent bongkrekic acid decreased apoptosis indicating that loss of ΔΨM is critical for SapC-DOPS activity. Apoptosis induction was not critically dependent on reactive oxygen species (ROS) production and Cyclophilin D, since pretreatment with N-acetyl cysteine and cyclosporine A, respectively, did not prevent Smac or Cyto c release.
Conclusions: Taken together, our results indicate that SapC-DOPS acts through a mitochondria-mediated pathway accompanied by an early release of Smac and Bax. Specific tumor-targeting capacity and anticancer efficacy of SapC-DOPS supports its potential as a dual imaging and therapeutic agent in neuroblastoma therapy.
Figures





Similar articles
-
Smac induces cytochrome c release and apoptosis independently from Bax/Bcl-x(L) in a strictly caspase-3-dependent manner in human carcinoma cells.Oncogene. 2004 Jun 3;23(26):4523-35. doi: 10.1038/sj.onc.1207594. Oncogene. 2004. PMID: 15064710
-
Methyl antcinate A from Antrodia camphorata induces apoptosis in human liver cancer cells through oxidant-mediated cofilin- and Bax-triggered mitochondrial pathway.Chem Res Toxicol. 2010 Jul 19;23(7):1256-67. doi: 10.1021/tx100116a. Chem Res Toxicol. 2010. PMID: 20557081
-
Detection of cancer cells using SapC-DOPS nanovesicles.Mol Cancer. 2016 May 10;15(1):33. doi: 10.1186/s12943-016-0519-1. Mol Cancer. 2016. PMID: 27160923 Free PMC article. Review.
-
SapC-DOPS nanovesicles as targeted therapy for lung cancer.Mol Cancer Ther. 2015 Feb;14(2):491-8. doi: 10.1158/1535-7163.MCT-14-0661. Mol Cancer Ther. 2015. PMID: 25670331 Free PMC article.
-
Saposin C-dioleylphosphatidylserine nanovesicles coupled with iron oxides.2011 Jun 20 [updated 2011 Jul 18]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Jun 20 [updated 2011 Jul 18]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21796836 Free Books & Documents. Review.
Cited by
-
Mitochondria mediates caspase-dependent and independent retinal cell death in Staphylococcus aureus endophthalmitis.Cell Death Discov. 2016 May 30;2:16034. doi: 10.1038/cddiscovery.2016.34. eCollection 2016. Cell Death Discov. 2016. PMID: 27551524 Free PMC article.
-
Phosphatidylserine: The Unique Dual-Role Biomarker for Cancer Imaging and Therapy.Cancers (Basel). 2022 May 21;14(10):2536. doi: 10.3390/cancers14102536. Cancers (Basel). 2022. PMID: 35626139 Free PMC article. Review.
-
Reuse of Molecules for Glioblastoma Therapy.Pharmaceuticals (Basel). 2021 Jan 28;14(2):99. doi: 10.3390/ph14020099. Pharmaceuticals (Basel). 2021. PMID: 33525329 Free PMC article. Review.
-
"Eat me" imaging and therapy.Adv Drug Deliv Rev. 2016 Apr 1;99(Pt A):2-11. doi: 10.1016/j.addr.2016.01.009. Epub 2016 Jan 27. Adv Drug Deliv Rev. 2016. PMID: 26826436 Free PMC article. Review.
-
Targeting of elevated cell surface phosphatidylserine with saposin C-dioleoylphosphatidylserine nanodrug as individual or combination therapy for pancreatic cancer.World J Gastrointest Oncol. 2021 Jun 15;13(6):550-559. doi: 10.4251/wjgo.v13.i6.550. World J Gastrointest Oncol. 2021. PMID: 34163572 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

