Anoikis-resistant subpopulations of human osteosarcoma display significant chemoresistance and are sensitive to targeted epigenetic therapies predicted by expression profiling
- PMID: 25889105
- PMCID: PMC4419490
- DOI: 10.1186/s12967-015-0466-4
Anoikis-resistant subpopulations of human osteosarcoma display significant chemoresistance and are sensitive to targeted epigenetic therapies predicted by expression profiling
Abstract
Background: Osteosarcoma (OS) is the most common type of solid bone cancer, with latent metastasis being a typical mode of disease progression and a major contributor to poor prognosis. For this to occur, cells must resist anoikis and be able to recapitulate tumorigenesis in a foreign microenvironment. Finding novel approaches to treat osteosarcoma and target those cell subpopulations that possess the ability to resist anoikis and contribute to metastatic disease is imperative. Here we investigate anchorage-independent (AI) cell growth as a model to better characterize anoikis resistance in human osteosarcoma while using an expression profiling approach to identify and test targetable signaling pathways.
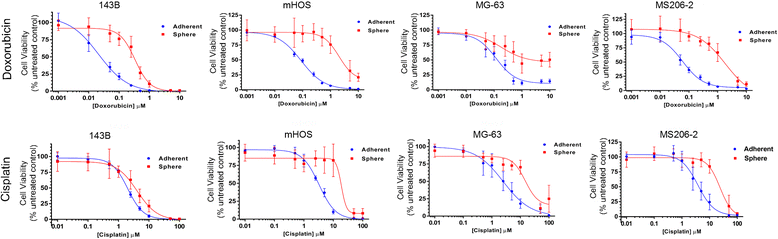
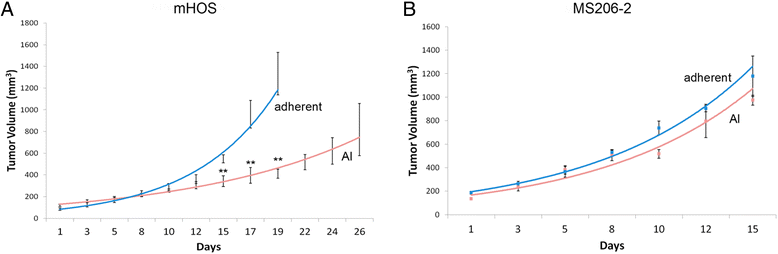
Methods: Established human OS cell lines and patient-derived human OS cell isolates were subjected to growth in either adherent or AI conditions using Ultra-Low Attachment plates in identical media conditions. Growth rate was assessed using cell doubling times and chemoresistance was assessed by determining cell viability in response to a serial dilution of either doxorubicin or cisplatin. Gene expression differences were examined using quantitative reverse-transcription PCR and microarray with principal component and pathway analysis. In-vivo OS xenografts were generated by either subcutaneous or intratibial injection of adherent or AI human OS cells into athymic nude mice. Statistical significance was determined using student's t-tests with significance set at α=0.05.
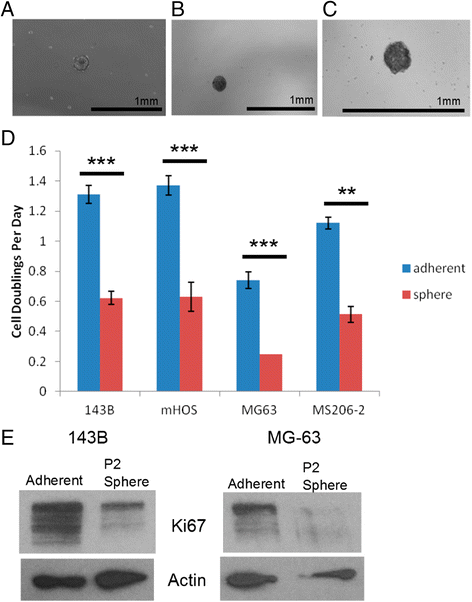
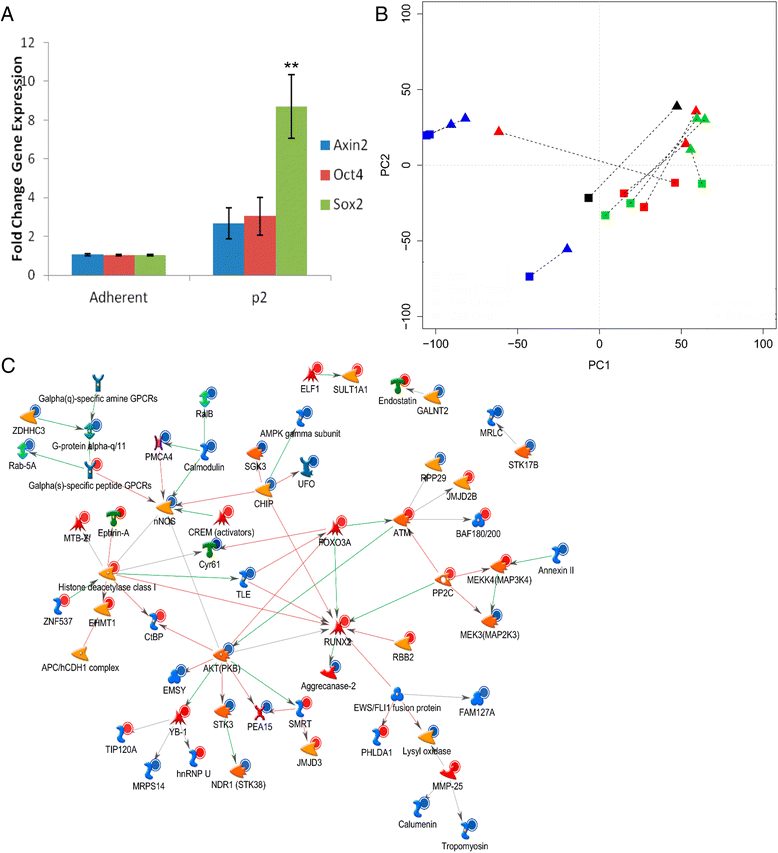
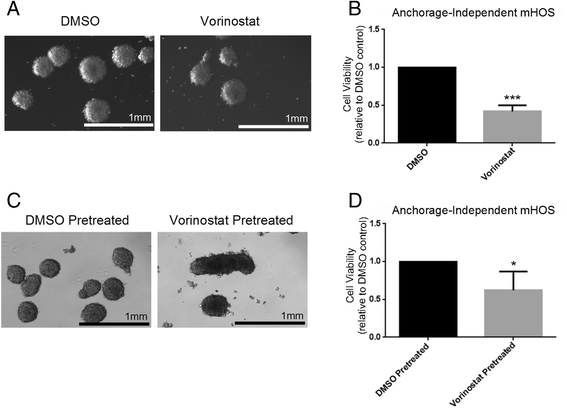
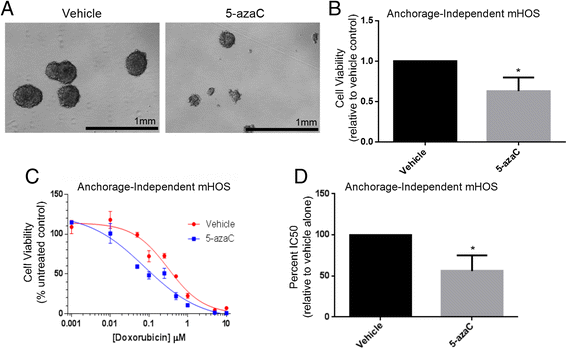
Results: We show that AI growth results in a global gene expression profile change accompanied by significant chemoresistance (up to 75 fold, p<0.05). AI cells demonstrate alteration of key mediators of mesenchymal differentiation (β-catenin, Runx2), stemness (Sox2), proliferation (c-myc, Akt), and epigenetic regulation (HDAC class 1). AI cells were equally tumorigenic as their adherent counterparts, but showed a significantly decreased rate of growth in-vitro and in-vivo (p<0.05). Treatment with the pan-histone deacetylase inhibitor vorinostat and the DNA methyltransferase inhibitor 5-azacytidine mitigated AI growth, while 5-azacytidine sensitized anoikis-resistant cells to doxorubicin (p<0.05).
Conclusions: These data demonstrate remarkable plasticity in anoikis-resistant human osteosarcoma subpopulations accompanied by a rapid development of chemoresistance and altered growth rates mirroring the early stages of latent metastasis. Targeting epigenetic regulation of this process may be a viable therapeutic strategy.
Figures
Similar articles
-
Long noncoding RNA expression profiles of the doxorubicin-resistant human osteosarcoma cell line MG63/DXR and its parental cell line MG63 as ascertained by microarray analysis.Int J Clin Exp Pathol. 2015 Aug 1;8(8):8754-73. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26464619 Free PMC article.
-
Tegavivint and the β-Catenin/ALDH Axis in Chemotherapy-Resistant and Metastatic Osteosarcoma.J Natl Cancer Inst. 2019 Nov 1;111(11):1216-1227. doi: 10.1093/jnci/djz026. J Natl Cancer Inst. 2019. PMID: 30793158 Free PMC article.
-
CXCR1/Akt signaling activation induced by mesenchymal stem cell-derived IL-8 promotes osteosarcoma cell anoikis resistance and pulmonary metastasis.Cell Death Dis. 2018 Jun 18;9(7):714. doi: 10.1038/s41419-018-0745-0. Cell Death Dis. 2018. PMID: 29915309 Free PMC article.
-
Long Non-coding RNAs in Cisplatin Resistance in Osteosarcoma.Curr Treat Options Oncol. 2021 Mar 20;22(5):41. doi: 10.1007/s11864-021-00839-y. Curr Treat Options Oncol. 2021. PMID: 33745006 Review.
-
Wnt signaling in osteosarcoma.Adv Exp Med Biol. 2014;804:33-45. doi: 10.1007/978-3-319-04843-7_2. Adv Exp Med Biol. 2014. PMID: 24924167 Review.
Cited by
-
TJP3 promotes T cell immunity escape and chemoresistance in breast cancer: a comprehensive analysis of anoikis-based prognosis prediction and drug sensitivity stratification.Aging (Albany NY). 2023 Nov 10;15(22):12890-12906. doi: 10.18632/aging.205208. Epub 2023 Nov 10. Aging (Albany NY). 2023. PMID: 37950731 Free PMC article.
-
Curcumin enhances chemotherapeutic effects and suppresses ANGPTL4 in anoikis-resistant cholangiocarcinoma cells.Heliyon. 2020 Jan 30;6(1):e03255. doi: 10.1016/j.heliyon.2020.e03255. eCollection 2020 Jan. Heliyon. 2020. PMID: 32051864 Free PMC article.
-
New anti-cancer chemicals Ertredin and its derivatives, regulate oxidative phosphorylation and glycolysis and suppress sphere formation in vitro and tumor growth in EGFRvIII-transformed cells.BMC Cancer. 2016 Jul 19;16:496. doi: 10.1186/s12885-016-2521-9. BMC Cancer. 2016. PMID: 27431653 Free PMC article.
-
Selective Targeting of Class I Histone Deacetylases in a Model of Human Osteosarcoma.Cancers (Basel). 2021 Aug 20;13(16):4199. doi: 10.3390/cancers13164199. Cancers (Basel). 2021. PMID: 34439353 Free PMC article.
-
Combination of Genomic Landsscape and 3D Culture Functional Assays Bridges Sarcoma Phenotype to Target and Immunotherapy.Cells. 2023 Sep 4;12(17):2204. doi: 10.3390/cells12172204. Cells. 2023. PMID: 37681936 Free PMC article. Review.
References
-
- Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–18. - PubMed
-
- Marcove RC, Mike V, Hajek JV, Levin AG, Hutter RV. Osteogenic sarcoma under the age of twenty-one. A review of one hundred and forty-five operative cases. J Bone Joint Surg Am. 1970;52:411–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

