Naturally-acquired cellular immune response against Plasmodium vivax merozoite surface protein-1 paralog antigen
- PMID: 25889175
- PMCID: PMC4403936
- DOI: 10.1186/s12936-015-0681-8
Naturally-acquired cellular immune response against Plasmodium vivax merozoite surface protein-1 paralog antigen
Abstract
Background: Plasmodium vivax merozoite surface protein-1 paralog (PvMSP1P) is a glycosylphosphatidylinositol-anchored protein expressed on the merozoite surface. This molecule is a target of natural immunity, as high anti-MSP1P-19 antibody levels were detected during P. vivax infection and the antibody inhibited PvMSP1P-erythrocyte binding. Recombinant PvMSP1P antigen results in production of a significant Th1 cytokine response in immunized mice. The present study was performed to characterize natural cellular immunity against PvMSP1P-19 and PvDBP region II in acute and recovery P. vivax infection.
Methods: Peripheral blood mononuclear cells (PBMCs) from acute and recovery P. vivax infection were obtained for lymphocyte proliferation assay upon PvMSP1P-19 and PvDBP region II antigen stimulation. The culture supernatant was examined for the presence of the cytokines IL-2, TNF, IFN-γ and IL-10 by enzyme-linked immunosorbent assay (ELISA). To determine whether Th1 or Th2 have a memory response against PvMSP1P-19 and PvDBPII protein antigen, PBMCs from subjects who had recovered from P. vivax infection 8-10 weeks prior to the study were obtained for lymphocyte proliferation assay. Cytokine-producing cells were analysed by flow cytometry.
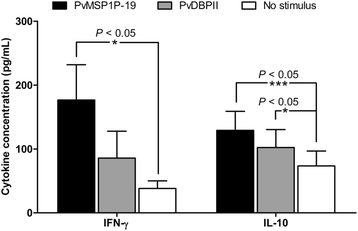
Results: IL-2 was detected at high levels in lymphocyte cultures from acutely infected P. vivax patients upon PvMSP1P-19 stimulation. Analysis of the Th1 or Th2 memory response in PBMC cultures from subjects who had recovered from P. vivax infection showed significantly elevated levels of PvMSP1P-19 and PvDBPII-specific IFN-γ-producing cells (P < 0.05). Interestingly, the response of IFN-γ-producing cells in PvMSP1P stimulation was fourfold greater in recovered subjects than that in acute-infection patients. CD4(+) T cells were the major cell phenotype involved in the response to PvMSP1P-19 and PvDBPII antigen.
Conclusions: PvMSP1P-19 strongly induces a specific cellular immune response for protection against P. vivax compared with PvDBPII as the antigen induces activation of IFN-γ-producing effector cells following natural P. vivax exposure. Upon stimulation, PvMSP1P-19 has the potential to activate the recall response of Th1 effector memory cells that play a role in killing the parasite.
Figures




References
-
- Mandal S. Epidemiological aspects of vivax and falciparum malaria: global spectrum. Asian Pacific J Trop Dis. 2014;4:S13–26. doi: 10.1016/S2222-1808(14)60410-2. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

