ATP synthesis is active on the cell surface of the shrimp Litopenaeus vannamei and is suppressed by WSSV infection
- PMID: 25889211
- PMCID: PMC4397868
- DOI: 10.1186/s12985-015-0275-7
ATP synthesis is active on the cell surface of the shrimp Litopenaeus vannamei and is suppressed by WSSV infection
Abstract
Background: Over the past a few years, evidences indicate that adenosine triphosphate (ATP) is an energy source for the binding, maturation, assembly, and budding process of many enveloped viruses. Our previous studies suggest that the F1-ATP synthase beta subunit (ATPsyn β, BP53) of the shrimp Litopenaeus vannamei (L. vannamei) might serve as a potential receptor for white spot syndrome virus (WSSV)'s infection.
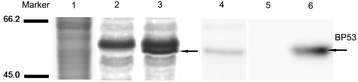
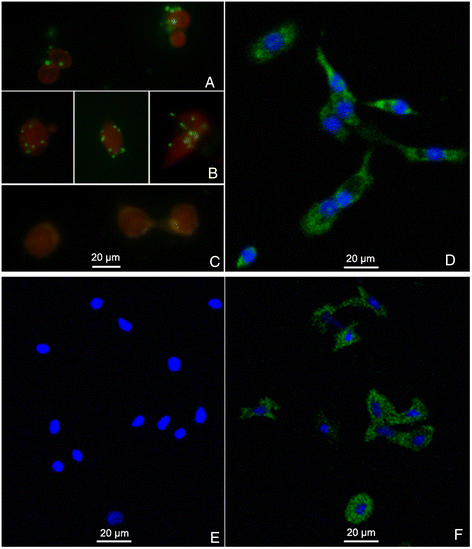
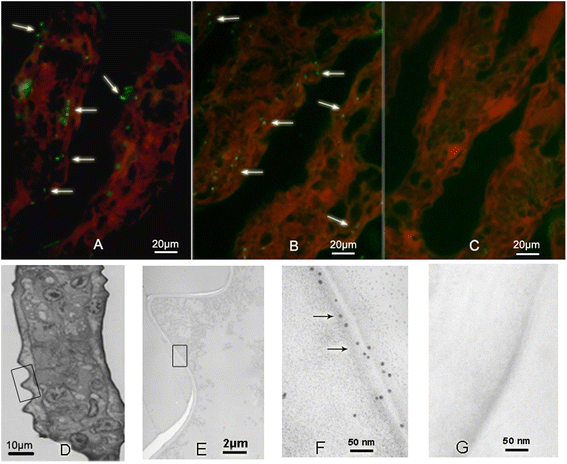
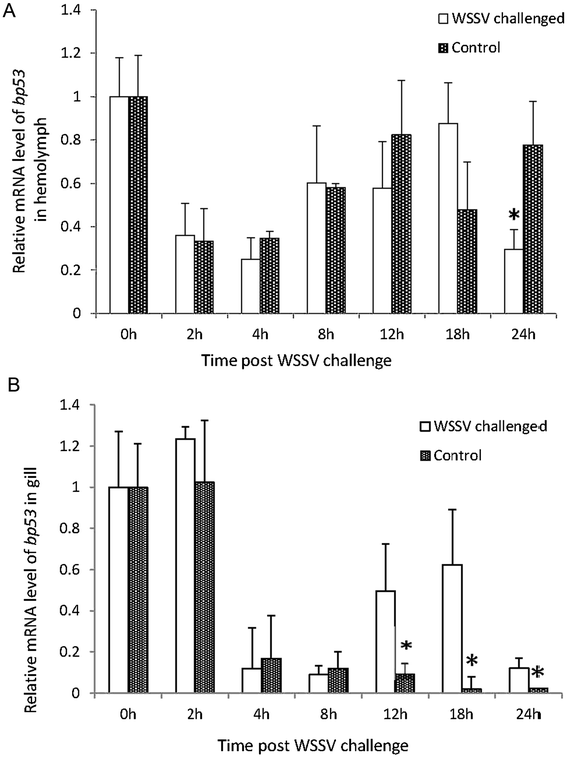
Methods: BP53 was localized on the surface of shrimp hemocytes and gill epithelial cells by immunofluorescence assay and immunogold labeling technique. Cell surface ATP synthesis was demonstrated by an in vitro bioluminescent luciferase assay. Furthermore, the expression of bp53 after WSSV infection was investigated by RT-PCR test. In addition, RNAi was developed to knock down endogenous bp53.
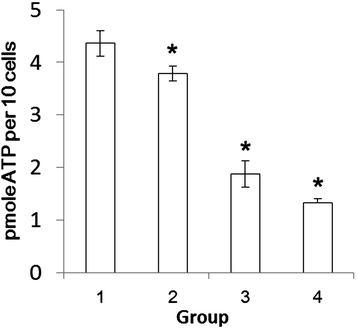
Results: BP53 is present on shrimp cell surface of hemocytes and gill epithelia. The synthesized ATP was detectable in the extracellular supernatant by using a bioluminescence assay, and the production declined post WSSV binding and infection. Knocking down endogenous bp53 resulted in a 50% mortality of L. vannamei.
Conclusion: These results suggested that BP53, presenting on cell surface, likely served as one of the receptors for WSSV infection in shrimp. Correspondingly, WSSV appears to disturb the host energy metabolism through interacting with host ATPsyn β during infection. This work firstly showed that host ATP production is required and consumed by the WSSV for binding and proceeds with infection process.
Figures

References
-
- Leu JH, Yang F, Zhang X, Xu X, Kou GH, Lo CF. Whispovirus. Curr Top Microbiol Immunol. 2009;328:197–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

