Patients lacking classical poor prognostic markers might also benefit from a step-down glucocorticoid bridging scheme in early rheumatoid arthritis: week 16 results from the randomized multicenter CareRA trial
- PMID: 25889222
- PMCID: PMC4422551
- DOI: 10.1186/s13075-015-0611-8
Patients lacking classical poor prognostic markers might also benefit from a step-down glucocorticoid bridging scheme in early rheumatoid arthritis: week 16 results from the randomized multicenter CareRA trial
Abstract
Introduction: Considering a lack of efficacy data in patients with early rheumatoid arthritis (eRA) presenting without classical markers of poor prognosis, we compared methotrexate (MTX) with or without step-down glucocorticoids in the CareRA trial.
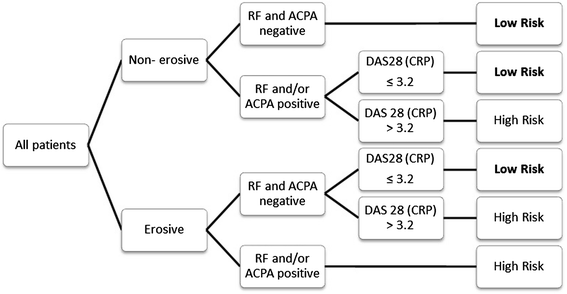
Methods: Disease-modifying antirheumatic drug-naïve patients with eRA were stratified into a low-risk group based on prognostic markers that included non-erosiveness, anti-citrullinated protein antibodies and rheumatoid factor negativity and low disease activity (Disease Activity Score in 28 joints based on C-reactive protein (DAS28(CRP)) ≤3.2). Patients were randomized to 15 mg of MTX weekly (MTX with tight step-up (MTX-TSU)) or 15 mg of MTX weekly with prednisone bridging, starting at 30 mg and tapered to 5 mg daily from week 6 (COmbinatie therapie bij Reumatoïde Artritis (COBRA Slim)). A TSU approach was applied. Outcomes assessed were DAS28(CRP)-determined remission, cumulative disease activity, Health Assessment Questionnaire (HAQ) scores and adverse events (AEs) after 16 treatment weeks.
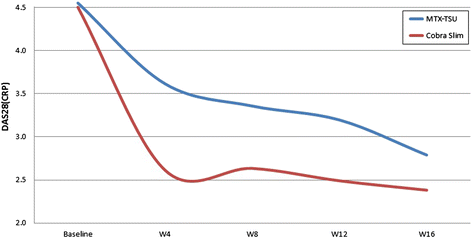
Results: We analyzed 43 COBRA Slim and 47 MTX-TSU patients and found that 65.1% in the COBRA Slim group and 46.8% in the MTX-TSU group reached remission (P = 0.081). Mean ± standard deviation area under the curve values of DAS28(CRP) were 13.84 ± 4.58 and 11.18 ± 4.25 for the MTX-TSU and COBRA Slim patients, respectively (P = 0.006). More COBRA Slim patients had an HAQ score of 0 (51.2% versus 23.4%, P = 0.006) at week 16. Therapy-related AEs between groups did not differ.
Conclusion: In patients with low-risk eRA, MTX with step-down glucocorticoid bridging seems more efficacious than MTX step-up monotherapy, with a comparable number of AEs observed over the first 16 treatment weeks.
Trial registration: EU Clinical Trials Register Identifier: EudraCT number 2008-007225-39 . Registered 5 November 2008.
Figures
References
-
- Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. doi: 10.1136/annrheumdis-2013-204573. - DOI - PMC - PubMed
-
- Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64:625–639. doi: 10.1002/acr.21641. - DOI - PMC - PubMed
-
- Boers M, Verhoeven AC, Markusse HM, van de Laar MA, Westhovens R, van Denderen JC, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997;350:309–18. A published erratum appears in. Lancet. 1998;351:220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

