Long lasting control of viral rebound with a new drug ABX464 targeting Rev - mediated viral RNA biogenesis
- PMID: 25889234
- PMCID: PMC4422473
- DOI: 10.1186/s12977-015-0159-3
Long lasting control of viral rebound with a new drug ABX464 targeting Rev - mediated viral RNA biogenesis
Abstract
Background: Current therapies have succeeded in controlling AIDS pandemic. However, there is a continuing need for new drugs, in particular those acting through new and as yet unexplored mechanisms of action to achieve HIV infection cure. We took advantage of the unique feature of proviral genome to require both activation and inhibition of splicing of viral transcripts to develop molecules capable of achieving long lasting effect on viral replication in humanized mouse models through inhibition of Rev-mediated viral RNA biogenesis.
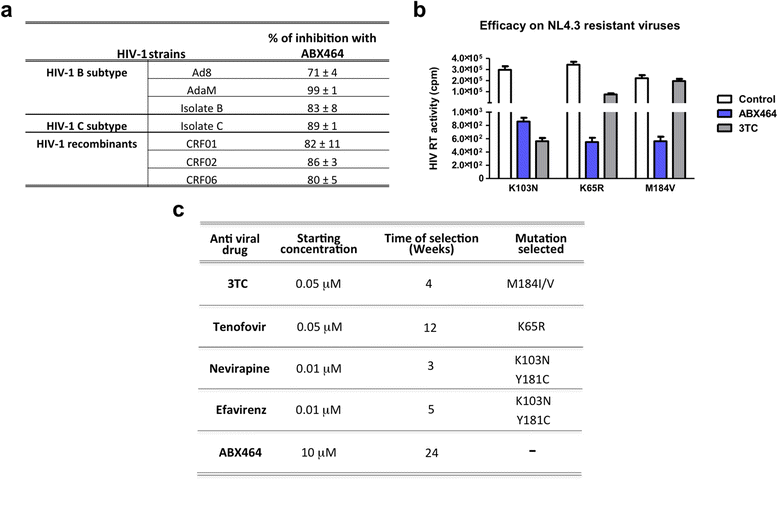
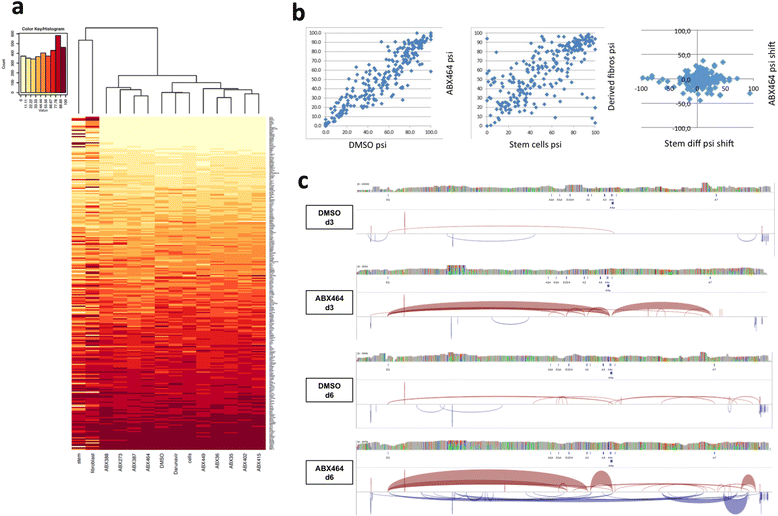
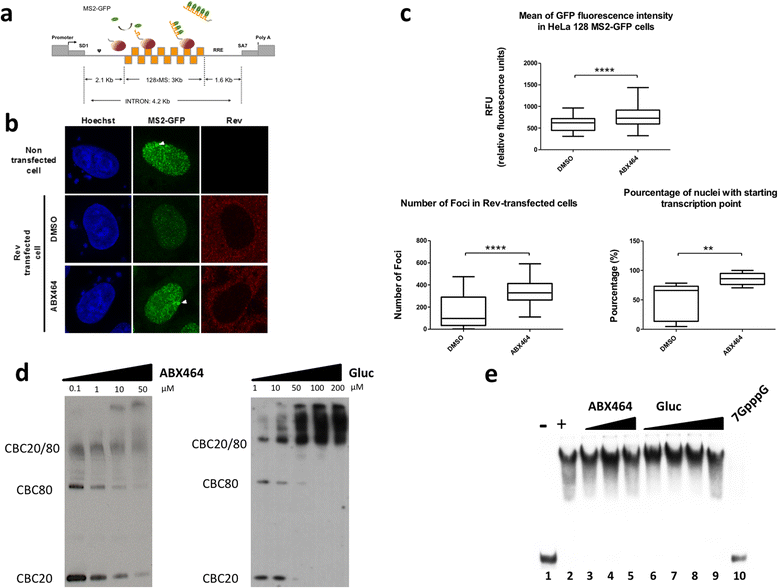
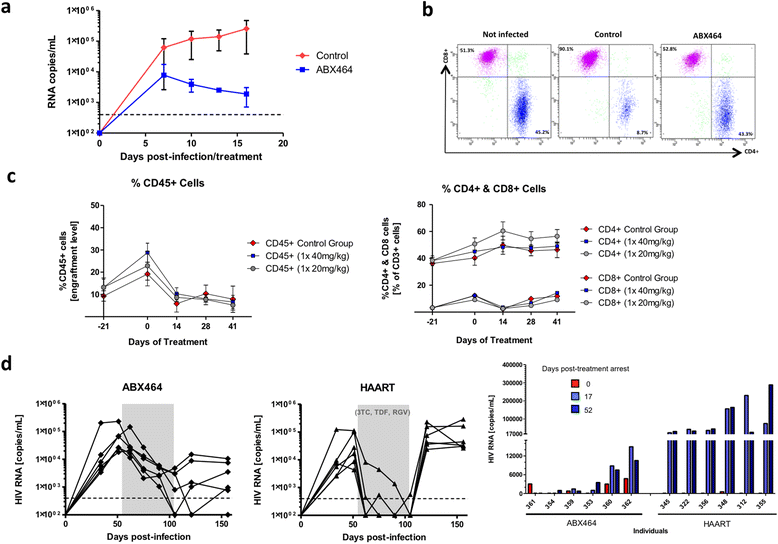
Results: Current HIV therapies reduce viral load during treatment but titers rebound after treatment is discontinued. We devised a new drug that has a long lasting effect after viral load reduction. We demonstrate here that ABX464 compromises HIV replication of clinical isolates of different subtypes without selecting for drug resistance in PBMCs or macrophages. ABX464 alone, also efficiently compromised viral proliferation in two humanized mouse models infected with HIV that require a combination of 3TC, Raltegravir and Tenofovir (HAART) to achieve viral inhibition in current protocols. Crucially, while viral load increased dramatically just one week after stopping HAART treatment, only slight rebound was observed following treatment cessation with ABX464 and the magnitude of the rebound was maintained below to that of HAART for two months after stopping the treatment. Using a system to visualize single HIV RNA molecules in living cells, we show that ABX464 inhibits viral replication by preventing Rev-mediated export of unspliced HIV-1 transcripts to the cytoplasm and by interacting with the Cap Binding Complex (CBC). Deep sequencing of viral RNA from treated cells established that retained viral RNA is massively spliced but importantly, normal cellular splicing is unaffected by the drug. Consistently ABX464 is non-toxic in humans and therefore represents a promising complement to current HIV therapies.
Conclusions: ABX464 represents a novel class of anti-HIV molecules with unique properties. ABX464 has a long lasting effect in humanized mice and neutralizes the expression of HIV-1 proviral genome of infected immune cells including reservoirs and it is therefore a promising drug toward a functional cure of HIV.
Figures

Comment in
-
ABX464: a good drug candidate instead of a magic bullet.Retrovirology. 2015 Jul 28;12:64. doi: 10.1186/s12977-015-0189-x. Retrovirology. 2015. PMID: 26215448 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

