miR-10b-5p expression in Huntington's disease brain relates to age of onset and the extent of striatal involvement
- PMID: 25889241
- PMCID: PMC4349621
- DOI: 10.1186/s12920-015-0083-3
miR-10b-5p expression in Huntington's disease brain relates to age of onset and the extent of striatal involvement
Abstract
Background: MicroRNAs (miRNAs) are small non-coding RNAs that recognize sites of complementarity of target messenger RNAs, resulting in transcriptional regulation and translational repression of target genes. In Huntington's disease (HD), a neurodegenerative disease caused by a trinucleotide repeat expansion, miRNA dyregulation has been reported, which may impact gene expression and modify the progression and severity of HD.
Methods: We performed next-generation miRNA sequence analysis in prefrontal cortex (Brodmann Area 9) from 26 HD, 2 HD gene positive, and 36 control brains. Neuropathological information was available for all HD brains, including age at disease onset, CAG-repeat size, Vonsattel grade, and Hadzi-Vonsattel striatal and cortical scores, a continuous measure of the extent of neurodegeneration. Linear models were performed to examine the relationship of miRNA expression to these clinical features, and messenger RNA targets of associated miRNAs were tested for gene ontology term enrichment.
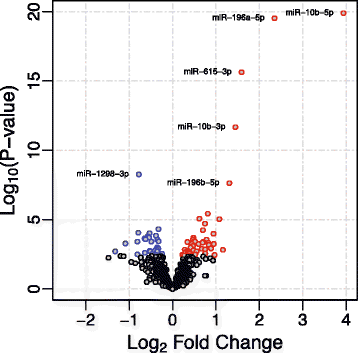
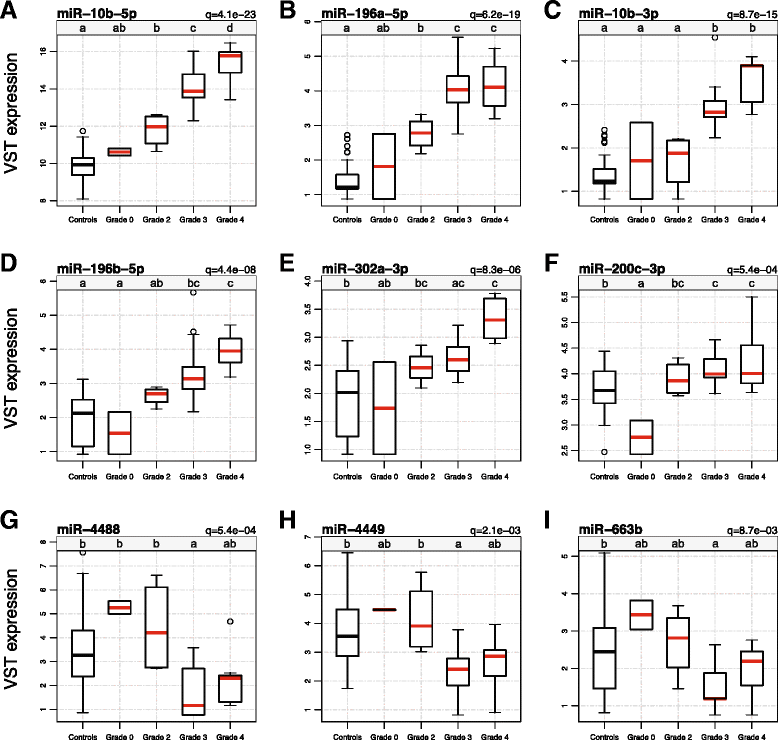
Results: We identified 75 miRNAs differentially expressed in HD brain (FDR q-value <0.05). Among the HD brains, nine miRNAs were significantly associated with Vonsattel grade of neuropathological involvement and three of these, miR-10b-5p, miR-10b-3p, and miR-302a-3p, significantly related to the Hadzi-Vonsattel striatal score (a continuous measure of striatal involvement) after adjustment for CAG length. Five miRNAs (miR-10b-5p, miR-196a-5p, miR-196b-5p, miR-10b-3p, and miR-106a-5p) were identified as having a significant relationship to CAG length-adjusted age of onset including miR-10b-5p, the mostly strongly over-expressed miRNA in HD cases. Although prefrontal cortex was the source of tissue profiled in these studies, the relationship of miR-10b-5p expression to striatal involvement in the disease was independent of cortical involvement. Correlation of miRNAs to the clinical features clustered by direction of effect and the gene targets of the observed miRNAs showed association to processes relating to nervous system development and transcriptional regulation.
Conclusions: These results demonstrate that miRNA expression in cortical BA9 provides insight into striatal involvement and support a role for these miRNAs, particularly miR-10b-5p, in HD pathogenicity. The miRNAs identified in our studies of postmortem brain tissue may be detectable in peripheral fluids and thus warrant consideration as accessible biomarkers for disease stage, rate of progression, and other important clinical characteristics of HD.
Figures



Similar articles
-
MicroRNAs located in the Hox gene clusters are implicated in huntington's disease pathogenesis.PLoS Genet. 2014 Feb 27;10(2):e1004188. doi: 10.1371/journal.pgen.1004188. eCollection 2014 Feb. PLoS Genet. 2014. PMID: 24586208 Free PMC article.
-
Circulating microRNAs in Huntington's disease: Emerging mediators in metabolic impairment.Pharmacol Res. 2016 Jun;108:102-110. doi: 10.1016/j.phrs.2016.05.005. Epub 2016 May 4. Pharmacol Res. 2016. PMID: 27155059
-
Down-regulation of miR-9* in the peripheral leukocytes of Huntington's disease patients.Orphanet J Rare Dis. 2017 Dec 19;12(1):185. doi: 10.1186/s13023-017-0742-x. Orphanet J Rare Dis. 2017. PMID: 29258536 Free PMC article.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
Transcriptional dysregulation of coding and non-coding genes in cellular models of Huntington's disease.Biochem Soc Trans. 2009 Dec;37(Pt 6):1270-5. doi: 10.1042/BST0371270. Biochem Soc Trans. 2009. PMID: 19909260 Review.
Cited by
-
Recent Advances in the Roles of MicroRNA and MicroRNA-Based Diagnosis in Neurodegenerative Diseases.Biosensors (Basel). 2022 Nov 24;12(12):1074. doi: 10.3390/bios12121074. Biosensors (Basel). 2022. PMID: 36551041 Free PMC article. Review.
-
Epigenetic mechanisms of neurodegenerative diseases and acute brain injury.Neurochem Int. 2020 Feb;133:104642. doi: 10.1016/j.neuint.2019.104642. Epub 2019 Dec 12. Neurochem Int. 2020. PMID: 31838024 Free PMC article. Review.
-
The Genomic Origins of Small Mitochondrial RNAs: Are They Transcribed by the Mitochondrial DNA or by Mitochondrial Pseudogenes within the Nucleus (NUMTs)?Genome Biol Evol. 2019 Jul 1;11(7):1883-1896. doi: 10.1093/gbe/evz132. Genome Biol Evol. 2019. PMID: 31218347 Free PMC article.
-
An in silico study to find potential effective circRNAs in the progression of Huntington's disease.Iran J Basic Med Sci. 2023;26(8):934-940. doi: 10.22038/IJBMS.2023.67791.14839. Iran J Basic Med Sci. 2023. PMID: 37427327 Free PMC article.
-
MicroRNA Expression and Neurocognitive Outcomes in Children and Young People With Primary Brain Tumor in Karachi, Pakistan: A Pilot Exploratory Study.Brain Tumor Res Treat. 2025 Jul;13(3):87-94. doi: 10.14791/btrt.2025.0006. Brain Tumor Res Treat. 2025. PMID: 40759476 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

