Interactions of highly and low virulent Flavobacterium columnare isolates with gill tissue in carp and rainbow trout
- PMID: 25889257
- PMCID: PMC4350652
- DOI: 10.1186/s13567-015-0164-5
Interactions of highly and low virulent Flavobacterium columnare isolates with gill tissue in carp and rainbow trout
Abstract
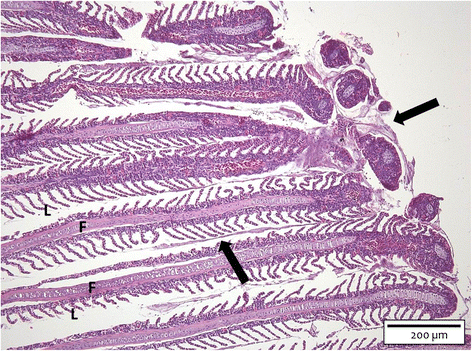
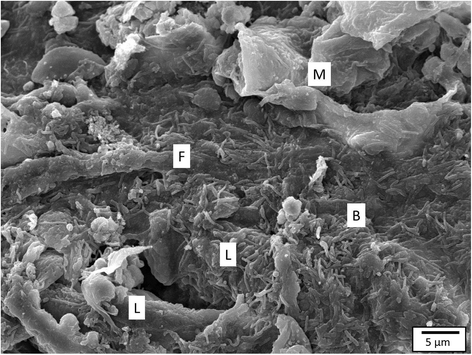
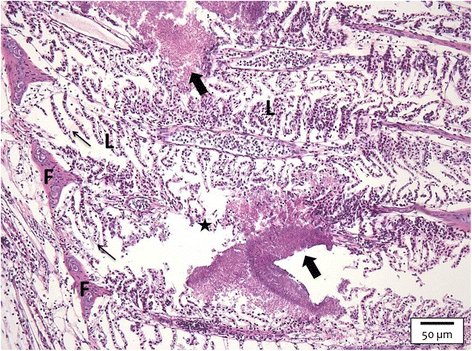
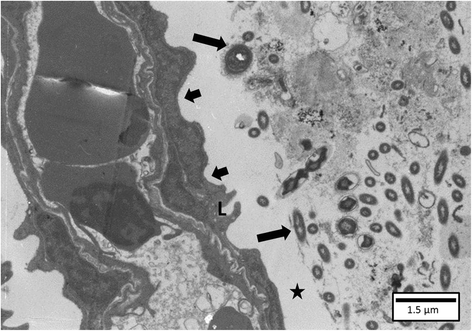
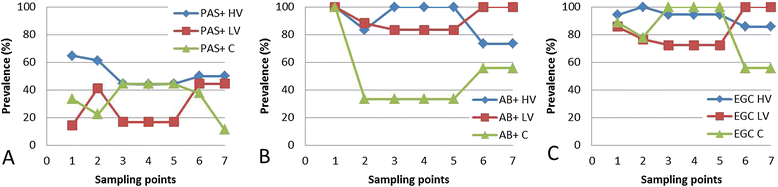
The interactions of Flavobacterium columnare isolates of different virulence with the gills of carp (Cyprinus carpio L.) and rainbow trout (Oncorhynchus mykiss Walbaum) were investigated. Both fish species were exposed to different high (HV) or low virulence (LV) isolates and sacrificed at seven predetermined times post-challenge. Histopathological and ultrastructural examination of carp and rainbow trout inoculated with the HV-isolate disclosed bacterial invasion and concomitant destruction of the gill tissue, gradually spreading from the filament tips towards the base, with outer membrane vesicles surrounding most bacterial cells. In carp, 5-10% of the fish inoculated with the LV-isolate became moribund and their gill tissue displayed the same features as described for the HV-isolate, albeit to a lesser degree. The bacterial numbers retrieved from the gill tissue were significantly higher for HV- compared to LV-isolate challenged carp and rainbow trout. TUNEL-stained and caspase-3-immunostained gill sections demonstrated significantly higher apoptotic cell counts in carp and rainbow trout challenged with the HV-isolate compared to control animals. Periodic acid-Schiff/alcian blue staining demonstrated a significantly higher total gill goblet cell count for HV- and LV-isolate challenged compared to control carp. Moreover, bacterial clusters were embedded in a neutral matrix while being encased by acid mucins, resembling biofilm formation. Eosinophilic granular cell counts were significantly higher in the HV-isolate compared to LV-isolate inoculated and control carp. The present data indicate a high colonization capacity, and the destructive and apoptotic-promoting features of the HV-isolate, and point towards important dynamic host mucin-F. columnare interactions warranting further research.
Figures





Similar articles
-
Gill infection model for columnaris disease in common carp and rainbow trout.J Aquat Anim Health. 2015 Mar;27(1):1-11. doi: 10.1080/08997659.2014.953265. J Aquat Anim Health. 2015. PMID: 25488182
-
Reproducible challenge model to investigate the virulence of Flavobacterium columnare genomovars in rainbow trout Oncorhynchus mykiss.Dis Aquat Organ. 2012 Nov 8;101(2):115-22. doi: 10.3354/dao02522. Dis Aquat Organ. 2012. PMID: 23135138
-
Virulence of Flavobacterium columnare genomovars in rainbow trout Oncorhynchus mykiss.Dis Aquat Organ. 2016 Aug 9;120(3):217-24. doi: 10.3354/dao03027. Dis Aquat Organ. 2016. PMID: 27503917
-
Flavobacterium columnare infections in fish: the agent and its adhesion to the gill tissue.Verh K Acad Geneeskd Belg. 2002;64(6):421-30. Verh K Acad Geneeskd Belg. 2002. PMID: 12649933 Review.
-
Columnaris disease in fish: a review with emphasis on bacterium-host interactions.Vet Res. 2013 Apr 24;44(1):27. doi: 10.1186/1297-9716-44-27. Vet Res. 2013. PMID: 23617544 Free PMC article. Review.
Cited by
-
Type IX Secretion System Effectors and Virulence of the Model Flavobacterium columnare Strain MS-FC-4.Appl Environ Microbiol. 2022 Feb 8;88(3):e0170521. doi: 10.1128/AEM.01705-21. Epub 2021 Nov 24. Appl Environ Microbiol. 2022. PMID: 34818105 Free PMC article.
-
Prevailing Role of Mucosal Igs and B Cells in Teleost Skin Immune Responses to Bacterial Infection.J Immunol. 2021 Mar 1;206(5):1088-1101. doi: 10.4049/jimmunol.2001097. Epub 2021 Jan 25. J Immunol. 2021. PMID: 33495235 Free PMC article.
-
Comparative genomics of Flavobacterium columnare unveils novel insights in virulence and antimicrobial resistance mechanisms.Vet Res. 2021 Feb 12;52(1):18. doi: 10.1186/s13567-021-00899-w. Vet Res. 2021. PMID: 33579339 Free PMC article.
-
Cortisol directly impacts Flavobacterium columnare in vitro growth characteristics.Vet Res. 2016 Aug 17;47(1):84. doi: 10.1186/s13567-016-0370-9. Vet Res. 2016. PMID: 27530746 Free PMC article.
-
Profilings of MicroRNAs in the Liver of Common Carp (Cyprinus carpio) Infected with Flavobacterium columnare.Int J Mol Sci. 2016 Apr 15;17(4):566. doi: 10.3390/ijms17040566. Int J Mol Sci. 2016. PMID: 27092486 Free PMC article.
References
-
- Bernardet JF, Bowman JP. The genus Flavobacterium. In: Dworkin M, Falkow S, editors. The Prokaryotes: A Handbook on the Biology of Bacteria. LLC, New York: Deeply Rooting Bacteria. Springer Science + Business Media; 2006. pp. 481–531.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

