Cohort study of consistency between the compliance with guidelines for chemotherapy-induced nausea and vomiting and patient outcome
- PMID: 25889295
- PMCID: PMC4379596
- DOI: 10.1186/s40360-015-0005-1
Cohort study of consistency between the compliance with guidelines for chemotherapy-induced nausea and vomiting and patient outcome
Abstract
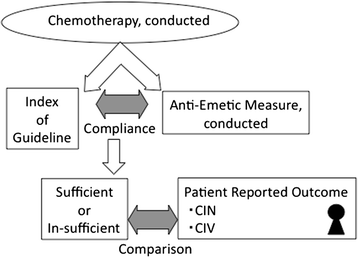
Background: Chemotherapy-induced nausea and vomiting is one of the most influential factors that affect patient quality of life; thus, preventing this adverse event could lead to better patient outcome. Standard preventive guidelines for antiemetic treatment have already been established based on the emetogenicity of chemotherapeutic agents. It is important that compliance with in-house guidelines and their effect on patient outcome is monitored.
Methods: In 3 years since the Akita university hospital antiemetic guidelines were outlined, we assessed the incidence of chemotherapy-induced nausea and vomiting using the antiemesis tool of the Multinational Association of Supportive Care in Cancer. Compliance of the guidelines was extracted from the hospital clinical record, and the chemotherapy-induced nausea and vomiting was examined by the patient reported outcome.
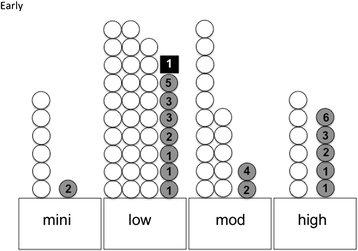
Results: Seventy-three patients answered the questionnaire. The overall compliance rate with the guidelines for early nausea and vomiting was 98.6% and with the delayed nausea and vomiting was 87.7%. The complete response rate for the early and delayed chemotherapy-induced nausea and vomiting was 77.8% and 73.8%, respectively. The overall relative risk of early nausea and vomiting was 0.22 (P < 0.05), whereas the relative risk for delayed nausea and vomiting was 2.09 (P < 0.05). Breakthrough vomiting was observed in 3 cases in the low-risk group only. These data suggest that delayed nausea and vomiting is difficult to prevent, particularly in the low-risk group. Further, it seems that the individual sensitivity for emetogenicity might differ among patients.
Conclusions: In addition to standard prevention guidelines based on emetogenicity, individual care based on patient reports should be considered for the complete prevention of chemotherapy-induced nausea and vomiting.
Figures

Similar articles
-
Antiemetic effectiveness and nausea and vomiting incidence during capecitabine and oxaliplatin chemotherapy.Nurs Res. 2012 Nov-Dec;61(6):405-12. doi: 10.1097/NNR.0b013e3182691438. Nurs Res. 2012. PMID: 22960588
-
Evaluation of the compliance with antiemetic guidelines for prevention of chemotherapy-induced nausea and vomiting in patients with hematologic malignancy.Pharmazie. 2019 Apr 1;74(4):250-254. doi: 10.1691/ph.2019.8889. Pharmazie. 2019. PMID: 30940311
-
Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE Study.J Oncol Pract. 2014 Jan;10(1):68-74. doi: 10.1200/JOP.2012.000816. Epub 2013 Sep 24. J Oncol Pract. 2014. PMID: 24065402
-
Reviewing current and emerging antiemetics for chemotherapy-induced nausea and vomiting prophylaxis.Hosp Pract (1995). 2015;43(4):226-34. doi: 10.1080/21548331.2015.1077095. Epub 2015 Aug 26. Hosp Pract (1995). 2015. PMID: 26308912 Review.
-
Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: past, present, and future recommendations.Oncologist. 2007 Sep;12(9):1143-50. doi: 10.1634/theoncologist.12-9-1143. Oncologist. 2007. PMID: 17914084 Review.
Cited by
-
Auriculotherapy to control chemotherapy-induced nausea and vomiting in patients with cancer: protocol of a systematic review.Syst Rev. 2019 Aug 15;8(1):206. doi: 10.1186/s13643-019-1124-3. Syst Rev. 2019. PMID: 31416474 Free PMC article.
-
Differential clinical pharmacology of rolapitant in delayed chemotherapy-induced nausea and vomiting (CINV).Drug Des Devel Ther. 2017 Mar 24;11:947-954. doi: 10.2147/DDDT.S108872. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28392676 Free PMC article. Review.
-
Network pharmacology and experimental verification-based strategy to explore the underlying mechanism of Liu Jun An Wei formula in the treatment of gastrointestinal reactions caused by chemotherapy for colorectal cancer.Front Pharmacol. 2022 Sep 20;13:999115. doi: 10.3389/fphar.2022.999115. eCollection 2022. Front Pharmacol. 2022. PMID: 36204230 Free PMC article.
-
Certified nurse specialists in cancer nursing and prophylactic antiemetic prescription for chemotherapy patients.Support Care Cancer. 2022 Jul;30(7):5931-5937. doi: 10.1007/s00520-022-07019-0. Epub 2022 Apr 7. Support Care Cancer. 2022. PMID: 35391572
-
Outcomes of chemotherapy-induced nausea and vomiting guideline adherence in pediatric and adult patients: a systematic review.Support Care Cancer. 2024 Jun 24;32(7):455. doi: 10.1007/s00520-024-08623-y. Support Care Cancer. 2024. PMID: 38913170
References
-
- Richardson JL, Marks G, Levine A. The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J Clin Oncol. 1988;6:1746–52. - PubMed
-
- Aapro M, Fabi A, Nolè F, Medici M, Steger G, Bachmann C, et al. Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol. 2010;21:1083–8. doi: 10.1093/annonc/mdp584. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

