Mature oligodendrocytes actively increase in vivo cytoskeletal plasticity following CNS damage
- PMID: 25889302
- PMCID: PMC4404661
- DOI: 10.1186/s12974-015-0271-2
Mature oligodendrocytes actively increase in vivo cytoskeletal plasticity following CNS damage
Abstract
Background: Oligodendrocytes are myelinating cells of the central nervous system which support functionally, structurally, and metabolically neurons. Mature oligodendrocytes are generally believed to be mere targets of destruction in the context of neuroinflammation and tissue damage, but their real degree of in vivo plasticity has become a matter of debate. We thus investigated the in vivo dynamic, actin-related response of these cells under different kinds of demyelinating stress.
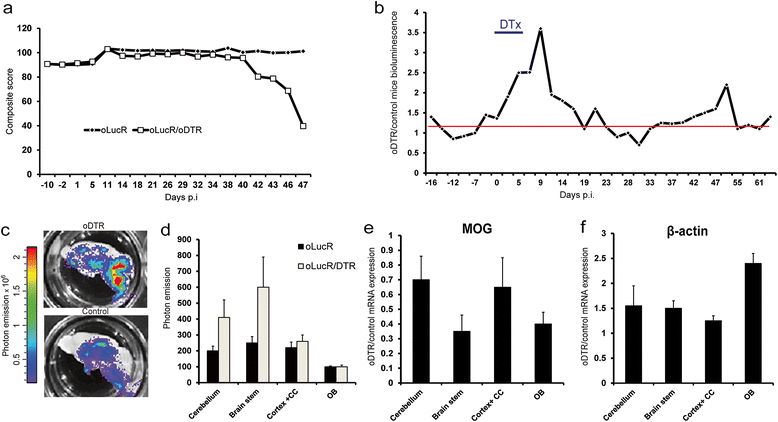
Methods: We used a novel mouse model (oLucR) expressing luciferase in myelin oligodendrocyte glycoprotein-positive oligodendrocytes under the control of a β-actin promoter. Activity of this promoter served as surrogate for dynamics of the cytoskeleton gene transcription through recording of in vivo bioluminescence following diphtheria toxin-induced oligodendrocyte death and autoimmune demyelination. Cytoskeletal gene expression was quantified from mature oligodendrocytes directly isolated from transgenic animals through cell sorting.
Results: Experimental demyelinating setups augmented oligodendrocyte-specific in vivo bioluminescence. These changes in luciferase signal were confirmed by further ex vivo analysis of the central nervous system tissue from oLucR mice. Increase in bioluminescence upon autoimmune inflammation was parallel to an oligodendrocyte-specific increased transcription of β-tubulin.
Conclusions: Mature oligodendrocytes acutely increase their cytoskeletal plasticity in vivo during demyelination. They are therefore not passive players under demyelinating conditions but can rather react dynamically to external insults.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

