Evaluation of the effect of tofacitinib on measured glomerular filtration rate in patients with active rheumatoid arthritis: results from a randomised controlled trial
- PMID: 25889308
- PMCID: PMC4445792
- DOI: 10.1186/s13075-015-0612-7
Evaluation of the effect of tofacitinib on measured glomerular filtration rate in patients with active rheumatoid arthritis: results from a randomised controlled trial
Abstract
Introduction: Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). During the clinical development programme, increases in mean serum creatinine (SCr) of approximately 0.07 mg/dL and 0.08 mg/dL were observed which plateaued early. This study assessed changes in measured glomerular filtration rate (mGFR) with tofacitinib relative to placebo in patients with active RA.
Methods: This was a randomised, placebo-controlled, Phase 1 study (NCT01484561). Patients were aged ≥18 years with active RA. Patients were randomised 2:1 to oral tofacitinib 10 mg twice daily (BID) in Period 1 then placebo BID in Period 2 (tofacitinib → placebo); or oral placebo BID in both Periods (placebo → placebo). Change in mGFR was evaluated by iohexol serum clearance at four time points (run-in, pre-dose in Period 1, Period 1 end, and Period 2 end). The primary endpoint was the change in mGFR from baseline to Period 1 end. Secondary endpoints included: change in mGFR at other time points; change in estimated GFR (eGFR; Cockcroft-Gault equation) and SCr; efficacy; and safety.
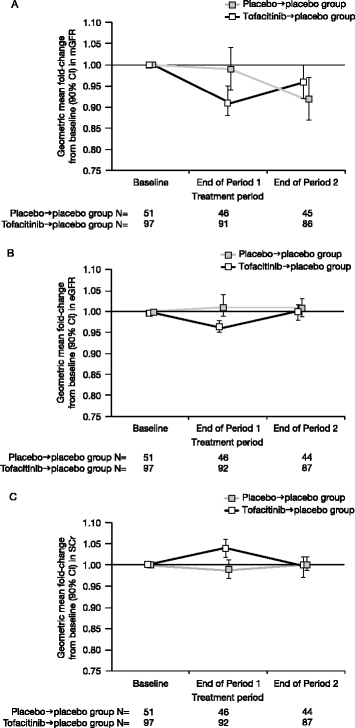
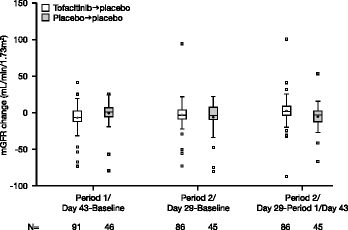
Results: 148 patients were randomised to tofacitinib → placebo (N = 97) or placebo → placebo (N = 51). Baseline characteristics were similar between groups. A reduction of 8% (90% confidence interval [CI]: 2%, 14%) from baseline in adjusted geometric mean mGFR was observed during tofacitinib treatment in Period 1 vs placebo. During Period 2, mean mGFR returned towards baseline during placebo treatment, and there was no difference between the two treatment groups at the end of the study--ratio (tofacitinib → placebo/placebo → placebo) of adjusted geometric mean fold change of mGFR was 1.04 (90% CI: 0.97, 1.11). Post-hoc analyses, focussed on mGFR variability in placebo → placebo patients, were consistent with this conclusion. At study end, similar results were observed for eGFR and SCr. Clinical efficacy and safety were consistent with prior studies.
Conclusion: Increases in mean SCr and decreases in eGFR in tofacitinib-treated patients with RA may occur in parallel with decreases in mean mGFR; mGFR returned towards baseline after tofacitinib discontinuation, with no significant difference vs placebo, even after post-hoc analyses. Safety monitoring will continue in ongoing and future clinical studies and routine pharmacovigilance.
Trial registration: Clinicaltrials.gov NCT01484561. Registered 30 November 2011.
Figures
References
-
- Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451–460. doi: 10.1016/S0140-6736(12)61424-X. - DOI - PubMed
-
- van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–570. doi: 10.1002/art.37816. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

