Interleukin-1 loop model for pathogenesis of Langerhans cell histiocytosis
- PMID: 25889448
- PMCID: PMC4343072
- DOI: 10.1186/s12964-015-0092-z
Interleukin-1 loop model for pathogenesis of Langerhans cell histiocytosis
Abstract
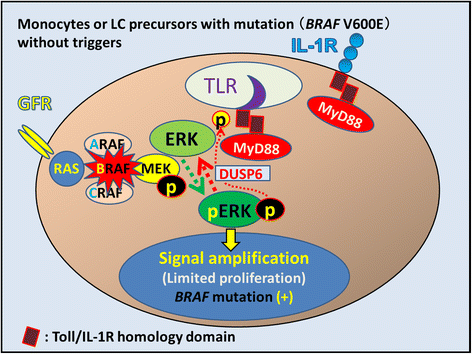
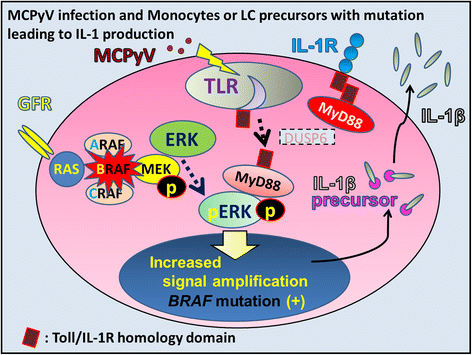
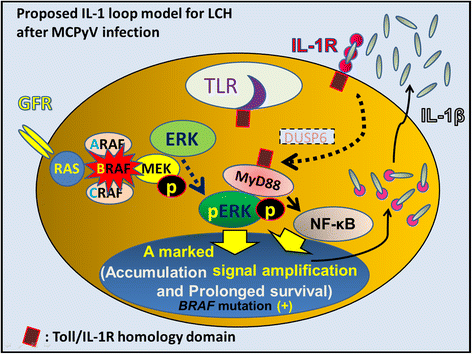
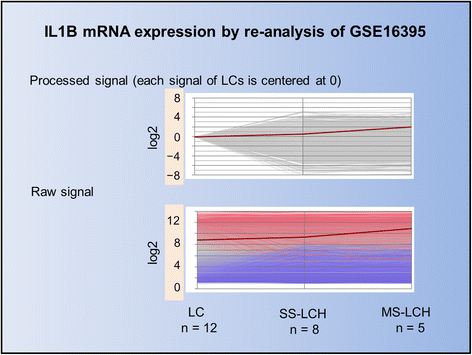
We propose Langerhans cell histiocytosis (LCH) is an inflammatory process that is prolonged by mutations. We hypothesize that Merkel cell polyomavirus (MCPyV) infection triggers an interleukin-1 (IL-1) activation loop that underlies the pathogenesis of LCH. Langerhans cells (LCs) are antigen presenting cells in the skin. When LCs encounter exogenous antigens, they migrate from the epidermis into draining lymphoid tissues to initiate T-cell activity. It has been proposed that LC migration-related factors, including E-cadherin, matrix metalloproteinase, and Notch ligand induce LCH activity. We found that the tyrosine phosphatase SHP-1, which binds IL-1 receptor-associated kinase 1, is expressed at a significantly higher level in LCH affecting multiple organ systems (MS-LCH) than in LCH affecting a single organ system (SS-LCH). IL-1 stimulates T helper 17 cells and their signature cytokine IL-17 had been a matter of controversy. We detected higher levels of IL-17A receptor expression in MS-LCH than in SS-LCH and proposed an IL-17 endocrine model that could settle the controversy. IL-1 is the first cytokine secreted in response to sensitizers and promotes LC migration from sentinel tissues. Myeloid differentiation primary response 88 (MyD88), downstream of the IL-1 receptor, has functions in both RAS signaling and inflammation, leading to human cell transformation. In 2010, an activating mutation in the B-rapidly accelerated fibrosarcoma gene (BRAF) V600E was found in LCH. This BRAF mutation induces phosphorylation of the extracellular signal-regulated kinase (ERK) that may play an important role with MyD88 in LCH pathogenesis. However, phosphorylated ERK (pERK) is rapidly dephosphorylated by dual specificity phosphatase 6 (DUSP6), and limited proliferation is predicted in BRAF mutant cells. MyD88 binds pERK via its D-domain, thereby preventing pERK-DUSP6 interaction and maintaining ERK in an active, phosphorylated state. We detected MCPyV-DNA in the peripheral blood cells of two out of three patients with LCH in high-risk organs but not in those of patients with LCH in non-high-risk organs (0/12; P = .029). MCPyV infection can trigger precursor LCH cells with BRAF mutation to produce IL-1; the IL-1 loop is amplified in all LCH subclasses. Our model indicates both BRAF mutation and IL-1 loop regulation as potential therapeutic targets.
Figures
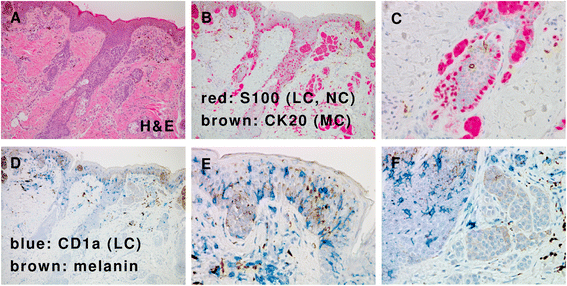
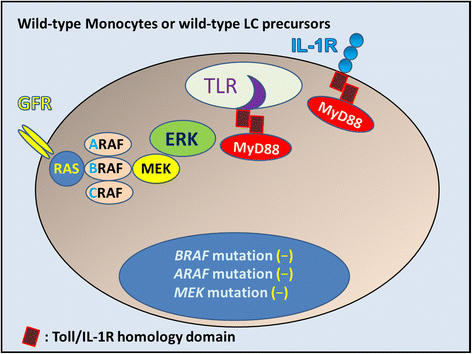
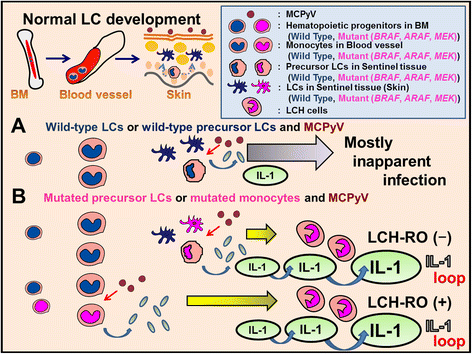

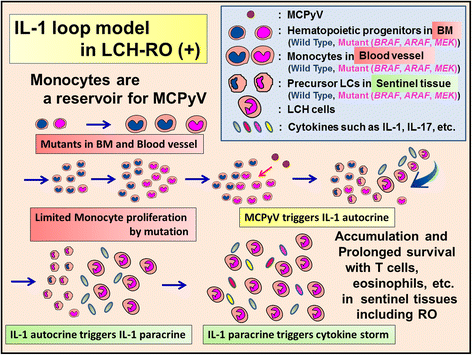
References
-
- Chu T, D’Angio GJ, Favara B, Ladisch S, Nesbit M, Pritchard J. Histiocytosis syndromes in children. Lancet. 1987;1:208–9. - PubMed
-
- Hamre M, Hedberg J, Buckley J, Bhatia S, Finlay J, Meadows A, et al. Langerhans cell histiocytosis: an exploratory epidemiologic study of 177 cases. Med Pediatr Oncol. 1997;28:92–7. - PubMed
-
- Weitzman S, Egeler RM. Histiocytic disorders of children and adults: introduction to the problem, overview, historical perspective and epidemiology. In: Weitzman S, Egeler RM, editors. Histiocytic Disorders of Children and Adults. Cambridge: Cambridge University Press; 2005. pp. 1–13.
-
- Salotti JA, Nanduri V, Pearce MS, Parker L, Lynn R, Windebank KP. Incidence and clinical features of Langerhans cell histiocytosis in the UK and Ireland. Arch Dis Child. 2009;94:376–80. - PubMed
-
- Alston RD, Tatevossian RG, McNally RJ, Kelsey A, Birch JM, Eden TO. Incidence and survival of childhood Langerhans cell histiocytosis in Northwest England from 1954 to 1998. Pediatr Blood Cancer. 2007;48:555–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

