TaqMan real-time polymerase chain reaction for detection of Ophidiomyces ophiodiicola, the fungus associated with snake fungal disease
- PMID: 25889462
- PMCID: PMC4404600
- DOI: 10.1186/s12917-015-0407-8
TaqMan real-time polymerase chain reaction for detection of Ophidiomyces ophiodiicola, the fungus associated with snake fungal disease
Abstract
Background: Fungal skin infections associated with Ophidiomyces ophiodiicola, a member of the Chrysosporium anamorph of Nannizziopsis vriesii (CANV) complex, have been linked to an increasing number of cases of snake fungal disease (SFD) in captive snakes around the world and in wild snake populations in eastern North America. The emergence of SFD in both captive and wild situations has led to an increased need for tools to better diagnose and study the disease.
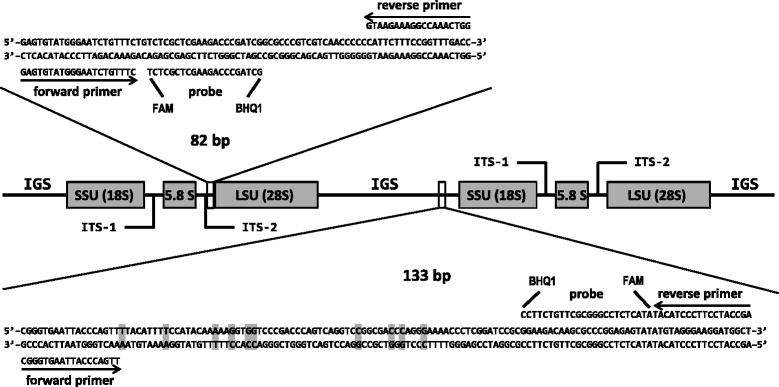
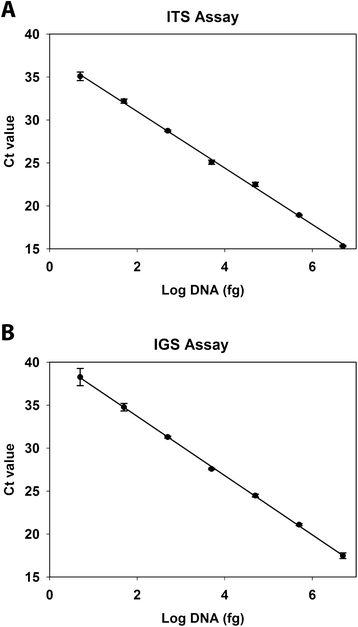
Results: We developed two TaqMan real-time polymerase chain reaction (PCR) assays to rapidly detect O. ophiodiicola in clinical samples. One assay targets the internal transcribed spacer region (ITS) of the fungal genome while the other targets the more variable intergenic spacer region (IGS). The PCR assays were qualified using skin samples collected from 50 snakes for which O. ophiodiicola had been previously detected by culture, 20 snakes with gross skin lesions suggestive of SFD but which were culture-negative for O. ophiodiicola, and 16 snakes with no clinical signs of infection. Both assays performed equivalently and proved to be more sensitive than traditional culture methods, detecting O. ophiodiicola in 98% of the culture-positive samples and in 40% of the culture-negative snakes that had clinical signs of SFD. In addition, the assays did not cross-react with a panel of 28 fungal species that are closely related to O. ophiodiicola or that commonly occur on the skin of snakes. The assays did, however, indicate that some asymptomatic snakes (~6%) may harbor low levels of the fungus, and that PCR should be paired with histology when a definitive diagnosis is required.
Conclusions: These assays represent the first published methods to detect O. ophiodiicola by real-time PCR. The ITS assay has great utility for assisting with SFD diagnoses whereas the IGS assay offers a valuable tool for research-based applications.
Figures
Similar articles
-
Experimental Infection of Snakes with Ophidiomyces ophiodiicola Causes Pathological Changes That Typify Snake Fungal Disease.mBio. 2015 Nov 17;6(6):e01534-15. doi: 10.1128/mBio.01534-15. mBio. 2015. PMID: 26578676 Free PMC article.
-
Emerging fungal pathogen Ophidiomyces ophiodiicola in wild European snakes.Sci Rep. 2017 Jun 19;7(1):3844. doi: 10.1038/s41598-017-03352-1. Sci Rep. 2017. PMID: 28630406 Free PMC article.
-
Snake fungal disease: an emerging threat to wild snakes.Philos Trans R Soc Lond B Biol Sci. 2016 Dec 5;371(1709):20150457. doi: 10.1098/rstb.2015.0457. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 28080983 Free PMC article. Review.
-
A Cross-Inoculation Experiment Reveals that Ophidiomyces ophiodiicola and Nannizziopsis guarroi Can Each Infect Both Snakes and Lizards.Appl Environ Microbiol. 2023 May 31;89(5):e0216822. doi: 10.1128/aem.02168-22. Epub 2023 Apr 26. Appl Environ Microbiol. 2023. PMID: 37098892 Free PMC article.
-
Chrysosporium anamorph Nannizziopsis vriesii: an emerging fungal pathogen of captive and wild reptiles.Vet Clin North Am Exot Anim Pract. 2013 Sep;16(3):659-68. doi: 10.1016/j.cvex.2013.05.013. Epub 2013 Jul 17. Vet Clin North Am Exot Anim Pract. 2013. PMID: 24018030 Review.
Cited by
-
Incidents of snake fungal disease caused by the fungal pathogen Ophidiomyces ophidiicola in Texas.Front Fungal Biol. 2023 Feb 23;4:1064939. doi: 10.3389/ffunb.2023.1064939. eCollection 2023. Front Fungal Biol. 2023. PMID: 37746129 Free PMC article.
-
Major Emerging Fungal Diseases of Reptiles and Amphibians.Pathogens. 2023 Mar 8;12(3):429. doi: 10.3390/pathogens12030429. Pathogens. 2023. PMID: 36986351 Free PMC article. Review.
-
Reproductive Traits and Hatchling Characteristics of the Endemic Sardinian Grass Snake (Natrix helvetica cetti): First Field Data, with Screening for Ophidiomyces ophidiicola.Animals (Basel). 2025 Feb 3;15(3):418. doi: 10.3390/ani15030418. Animals (Basel). 2025. PMID: 39943188 Free PMC article.
-
Snake Fungal Disease (Ophidiomycosis) in Northern Pine Snakes (Pituophis melanoleucus melanoleucus) in New Jersey: Variations by Year, Sex, and Morphological Sampling Site.J Fungi (Basel). 2025 Mar 6;11(3):206. doi: 10.3390/jof11030206. J Fungi (Basel). 2025. PMID: 40137244 Free PMC article.
-
Characterisation, prevalence and severity of skin lesions caused by ophidiomycosis in a population of wild snakes.Sci Rep. 2024 Mar 2;14(1):5162. doi: 10.1038/s41598-024-55354-5. Sci Rep. 2024. PMID: 38431688 Free PMC article.
References
-
- Hoppmann E, Barron HW. Dermatology in reptiles. J Exot Pet Med. 2007;16:210–24. doi: 10.1053/j.jepm.2007.10.001. - DOI
-
- Paré JA, Jacobson ER. Mycotic diseases of reptiles. In: Jacobson ER, editor. Infectious Diseases and Pathology of Reptiles: Color Atlas and Text. Boca Raton, FL: CRC Press, Taylor and Francis; 2007. pp. 527–70.
-
- Paré JA, Sigler L, Rypien KL, Gibas CC. Cutaneous mycobiota of captive squamate reptiles with notes on the scarcity of Chrysosporium anamorph of Nannizziopsis vriesii. J Herpetol Med Surg. 2003;13:10–5.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

