Kinetics of gene expression and bone remodelling in the clinical phase of collagen-induced arthritis
- PMID: 25889670
- PMCID: PMC4391727
- DOI: 10.1186/s13075-015-0531-7
Kinetics of gene expression and bone remodelling in the clinical phase of collagen-induced arthritis
Abstract
Introduction: Pathological bone changes differ considerably between inflammatory arthritic diseases and most studies have focused on bone erosion. Collagen-induced arthritis (CIA) is a model for rheumatoid arthritis, which, in addition to bone erosion, demonstrates bone formation at the time of clinical manifestations. The objective of this study was to use this model to characterise the histological and molecular changes in bone remodelling, and relate these to the clinical disease development.
Methods: A histological and gene expression profiling time-course study on bone remodelling in CIA was linked to onset of clinical symptoms. Global gene expression was studied with a gene chip array system.
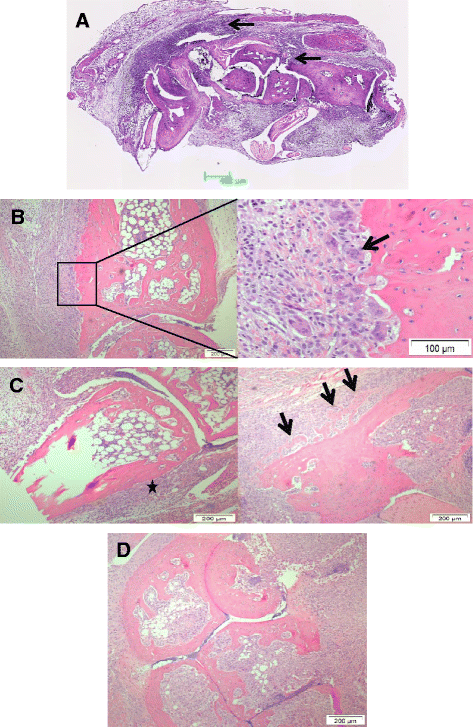
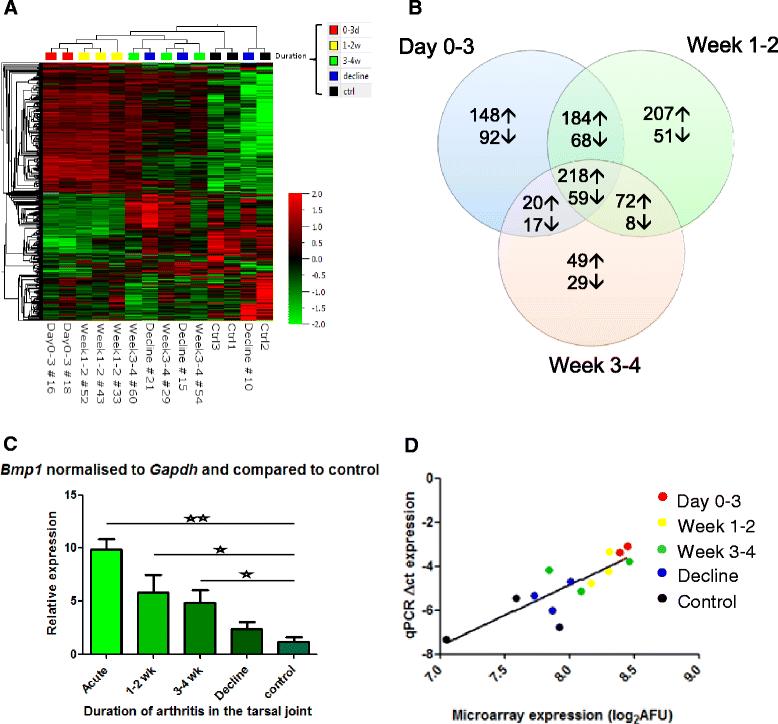
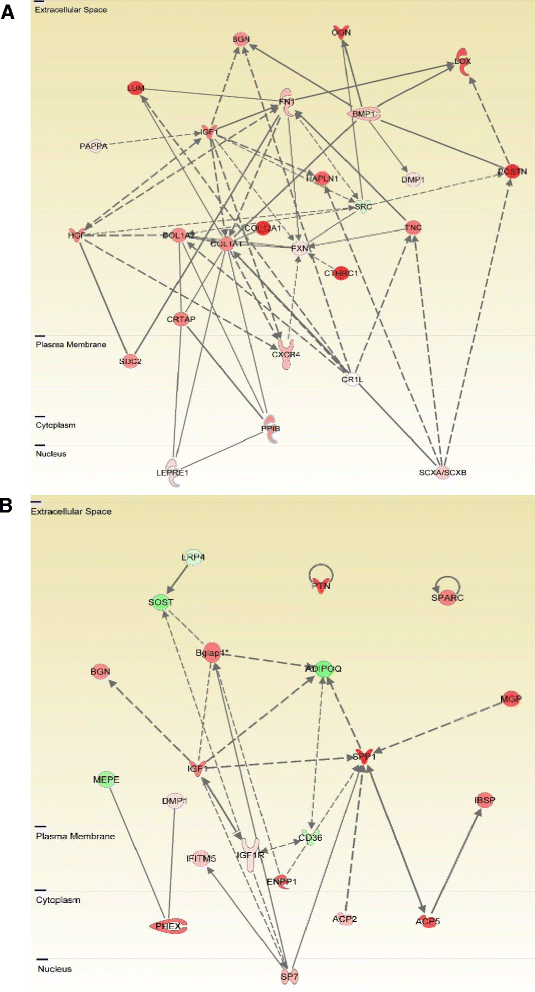
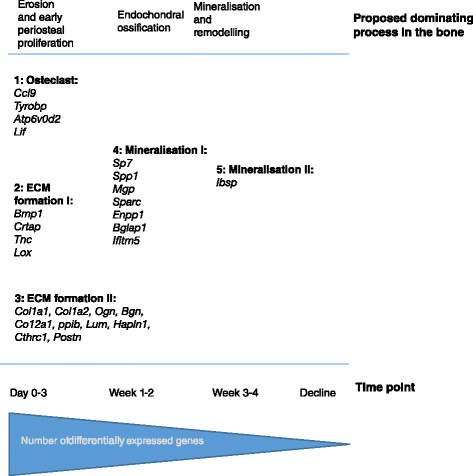
Results: The main histopathological changes in bone structure and inflammation occurred during the first two weeks following the onset of clinical symptoms in the joint. Hereafter, the inflammation declined and remodelling of formed bone dominated. Global gene expression profiling showed simultaneous upregulation of genes related to bone changes and inflammation in week 0 to 2 after onset of clinical disease. Furthermore, we observed time-dependent expression of genes involved in early and late osteoblast differentiation and function, which mirrored the histopathological bone changes. The differentially expressed genes belong to the bone morphogenetic pathway (BMP) and, in addition, include the osteoblast markers integrin-binding sialoprotein (Ibsp), bone gamma-carboxyglutamate protein (Bglap1), and secreted phosphoprotein 1 (Spp1). Pregnancy-associated protein A (Pappa) and periostin (Postn), differentially expressed in the early disease phase, are proposed to participate in bone formation, and we suggest that they play a role in early bone formation in the CIA model. Comparison to human genome-wide association studies (GWAS) revealed differential expression of several genes associated with human arthritis.
Conclusions: In the CIA model, bone formation in the joint starts shortly after onset of clinical symptoms, which results in bony fusion within one to two weeks. This makes it a candidate model for investigating the relationship between inflammation and bone formation in inflammatory arthritis.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

