Development of a simple procedure for the treatment of femoral head osteonecrosis with intra-osseous injection of bone marrow mesenchymal stromal cells: study of their biodistribution in the early time points after injection
- PMID: 25889681
- PMCID: PMC4448288
- DOI: 10.1186/s13287-015-0036-y
Development of a simple procedure for the treatment of femoral head osteonecrosis with intra-osseous injection of bone marrow mesenchymal stromal cells: study of their biodistribution in the early time points after injection
Abstract
Introduction: Osteonecrosis of the femoral head (ONFH) is a degenerative disease progressing to a femoral head (FH) collapse. Injection of osteoprogenitor cells like bone marrow mesenchymal stromal cells (BMSCs) into the FH appears to be a good therapeutic treatment. However, safety and efficacy of BMSCs to treat bone defect are the main preclinical data required for clinical application. Efficacy and the lack of risk of cell transformation after amplification of BMSCs have been extensively described. The main objectives of this study were to develop a simple and usable procedure for clinicians and control its feasibility by evaluating the biodistribution of BMSCs after injection into the FH in a large animal model. The impact of this approach was evaluated on one natural pig ONFH.
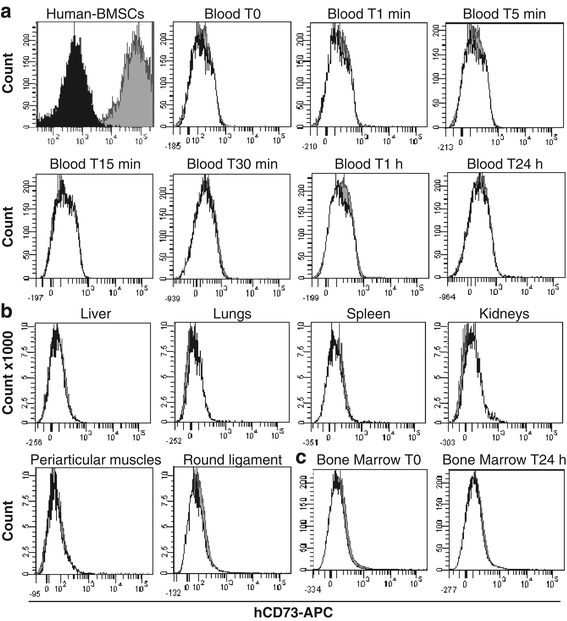
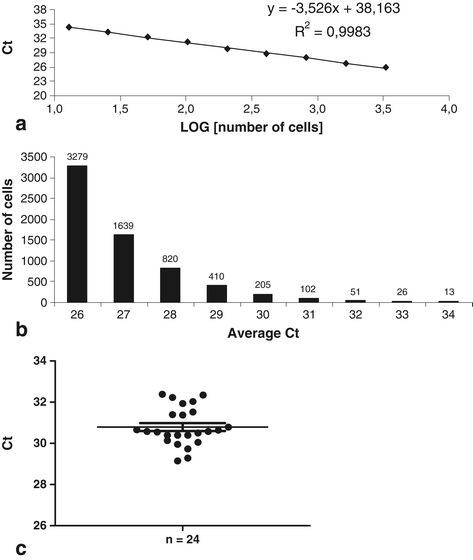
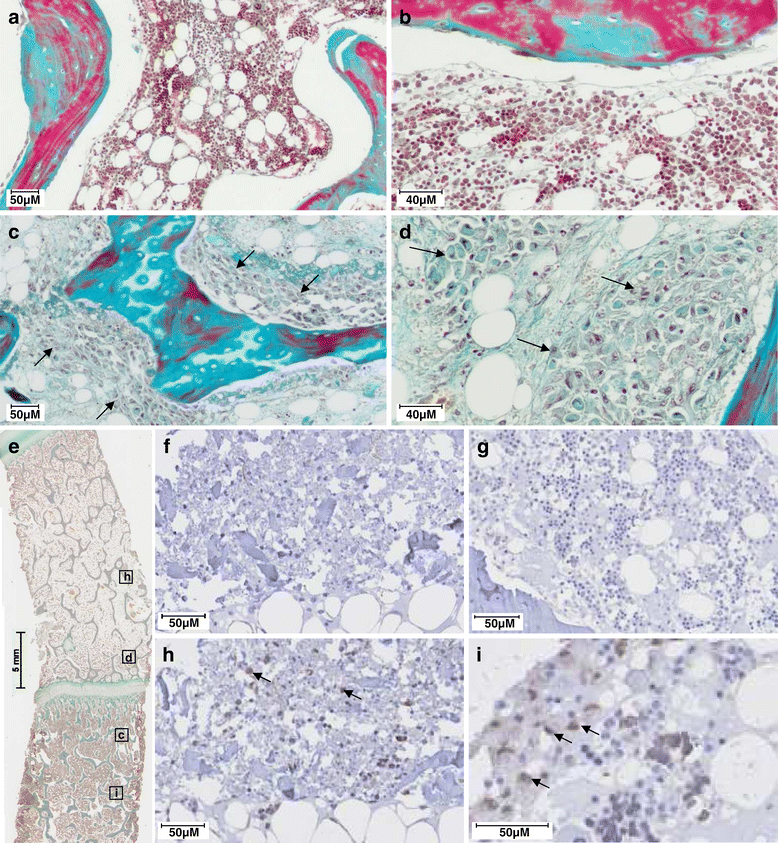
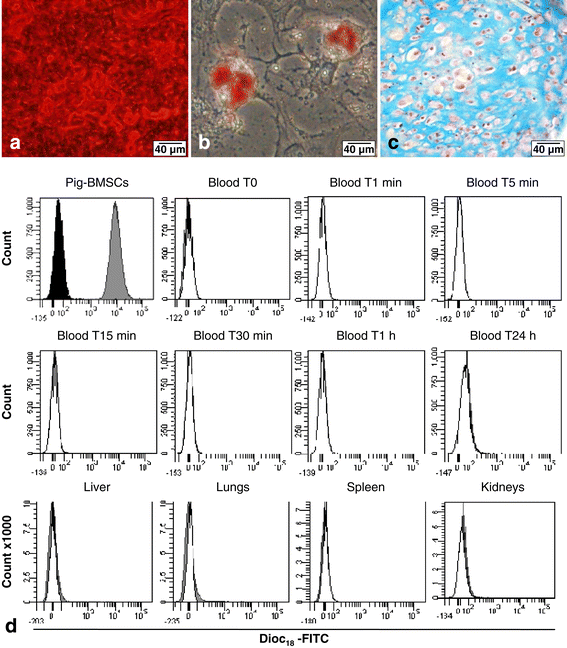
Methods: BMSCs were directly injected in the pig FH, and then the biodistribution of grafted cells was detected by quantitative real-time polymerase chain reaction, cytometry, or a combination of classic histology analysis and in situ hybridization (ISH). BMSC efficacy on bone regeneration was evaluated by magnetic resonance imaging (MRI) and histology.
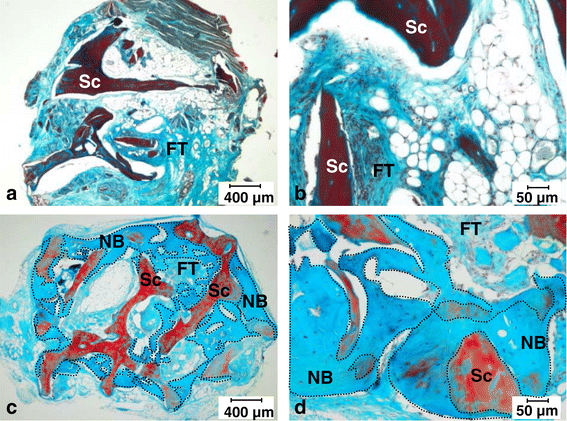
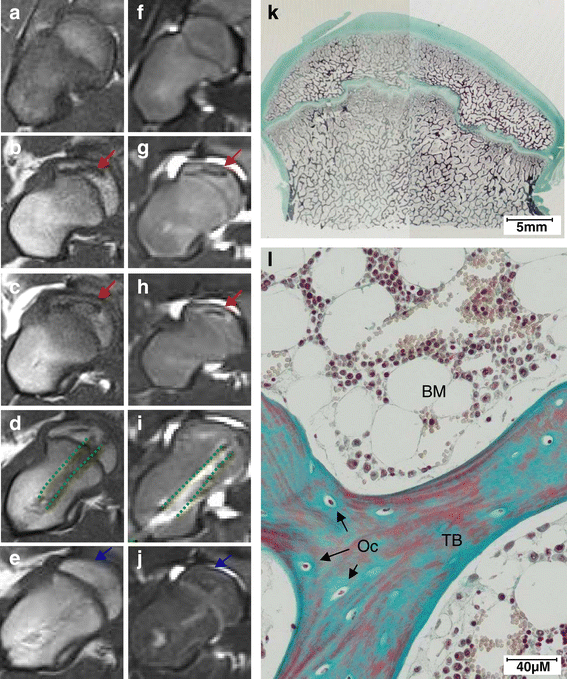
Results: After 30-minute and 24-hour follow-up, grafted cells were detected at the injection site and no BMSCs were detected in filter organs or body fluids. The combination of classic histology analysis and ISH showed a good homogeneity of cell distribution in FH. Local delivery of BMSCs onto a bone scaffold associated with bone formation in vivo confirmed the preferential tropism of BMSCs to the bone tissue as well as their efficacy to form bone. Treatment of a natural pig ONFH by autologous BMSCs indicated a beginning of bone healing as early as 2 weeks with a complete healing after 9 weeks. At this stage, MRI and histological analysis were similar to those of a normal FH.
Conclusions: Intra-osseous injection of BMSCs in FH seems to be a good strategy for ONFH treatment as the safety concerning the biodistribution of BMSCs is ensured. Moreover, the efficacy of BMSCs in natural ONFH seems to indicate that this is a promising approach. Altogether, these results constitute the preclinical data necessary for the setup of a clinical application with expanded BMSCs in the context of advanced therapy medicinal products.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

