Development of a computerised decision aid for thrombolysis in acute stroke care
- PMID: 25889696
- PMCID: PMC4326413
- DOI: 10.1186/s12911-014-0127-1
Development of a computerised decision aid for thrombolysis in acute stroke care
Abstract
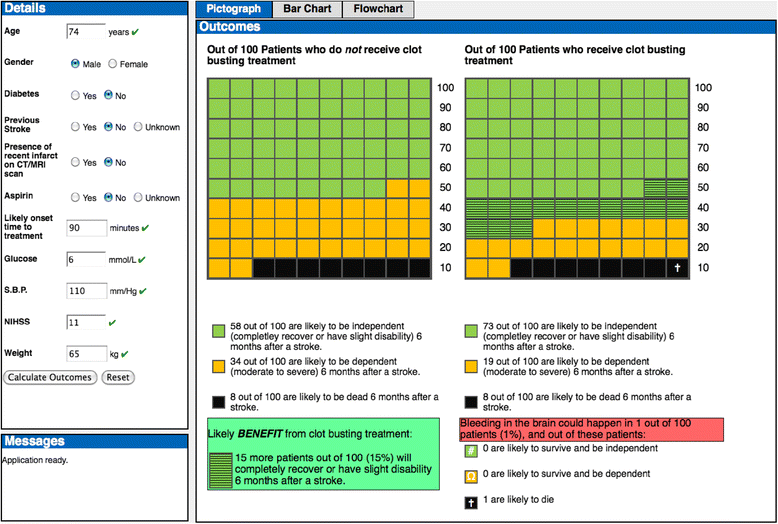
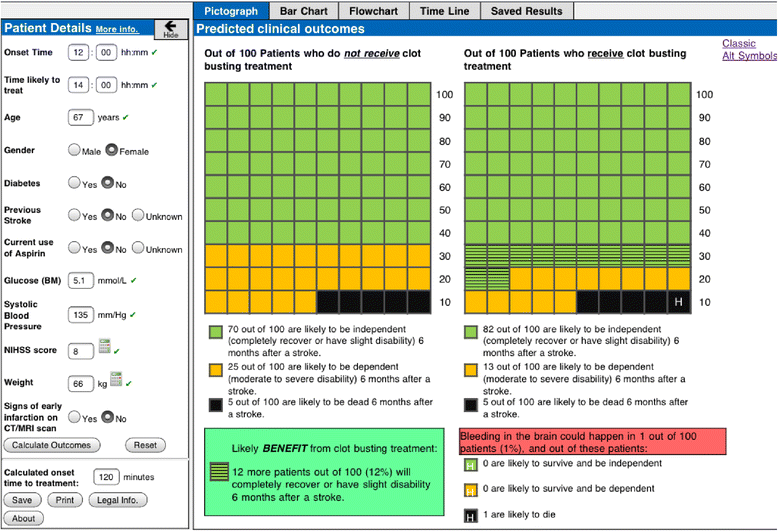
Background: Thrombolytic treatment for acute ischaemic stroke improves prognosis, although there is a risk of bleeding complications leading to early death/severe disability. Benefit from thrombolysis is time dependent and treatment must be administered within 4.5 hours from onset of symptoms, which presents unique challenges for development of tools to support decision making and patient understanding about treatment. Our aim was to develop a decision aid to support patient-specific clinical decision-making about thrombolysis for acute ischaemic stroke, and clinical communication of personalised information on benefits/risks of thrombolysis by clinicians to patients/relatives.
Methods: Using mixed methods we developed a COMPuterised decision Aid for Stroke thrombolysiS (COMPASS) in an iterative staged process (review of available tools; a decision analytic model; interactive group workshops with clinicians and patients/relatives; and prototype usability testing). We then tested the tool in simulated situations with final testing in real life stroke thrombolysis decisions in hospitals. Clinicians used COMPASS pragmatically in managing acute stroke patients potentially eligible for thrombolysis; their experience was assessed using self-completion forms and interviews. Computer logged data assessed time in use, and utilisation of graphical risk presentations and additional features. Patients'/relatives' experiences of discussions supported by COMPASS were explored using interviews.
Results: COMPASS expresses predicted outcomes (bleeding complications, death, and extent of disability) with and without thrombolysis, presented numerically (percentages and natural frequencies) and graphically (pictographs, bar graphs and flowcharts). COMPASS was used for 25 patients and no adverse effects of use were reported. Median time in use was 2.8 minutes. Graphical risk presentations were shared with 14 patients/relatives. Clinicians (n = 10) valued the patient-specific predictions of benefit from thrombolysis, and the support of better risk communication with patients/relatives. Patients (n = 2) and relatives (n = 6) reported that graphical risk presentations facilitated understanding of benefits/risks of thrombolysis. Additional features (e.g. dosage calculator) were suggested and subsequently embedded within COMPASS to enhance usability.
Conclusions: Our structured development process led to the development of a gamma prototype computerised decision aid. Initial evaluation has demonstrated reasonable acceptability of COMPASS amongst patients, relatives and clinicians. The impact of COMPASS on clinical outcomes requires wider prospective evaluation in clinical settings.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

