Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells
- PMID: 25889741
- PMCID: PMC4404686
- DOI: 10.1186/s13287-015-0013-5
Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells
Abstract
Introduction: Stem cells isolated from menstrual fluid (MenSCs) exhibit mesenchymal stem cell (MSCs)-like properties including multi-lineage differentiation capacity. Besides, menstrual fluid has important advantages over other sources for the isolation of MSCs, including ease of access and repeated sampling in a noninvasive manner. Such attributes allow the rapid culture of MenSCs in numbers that are sufficient for therapeutical doses, at lower cell passages.
Methods: In this study, we advance the characterization of MenSC populations in comparison to bone marrow derived mesenchymal stem cells (BM-MSCs) with regards to proliferation, lineage differentiation, migration potential, secretion profile and angiogenic properties in vitro and in a matrigel plug assay in mice. We additionally tested their ability to support hematopoietic stem cell (HSC) expansion in vitro.
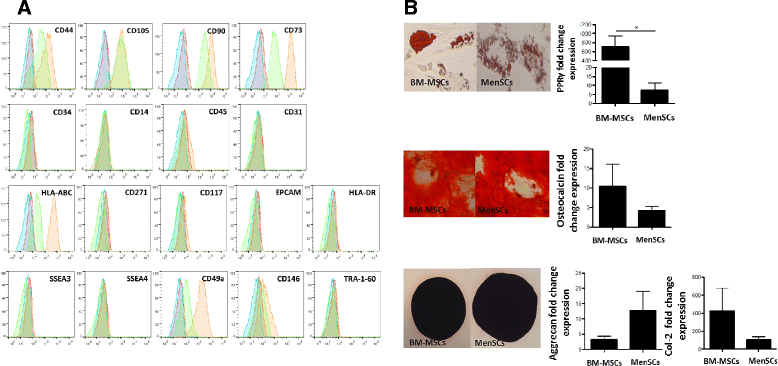
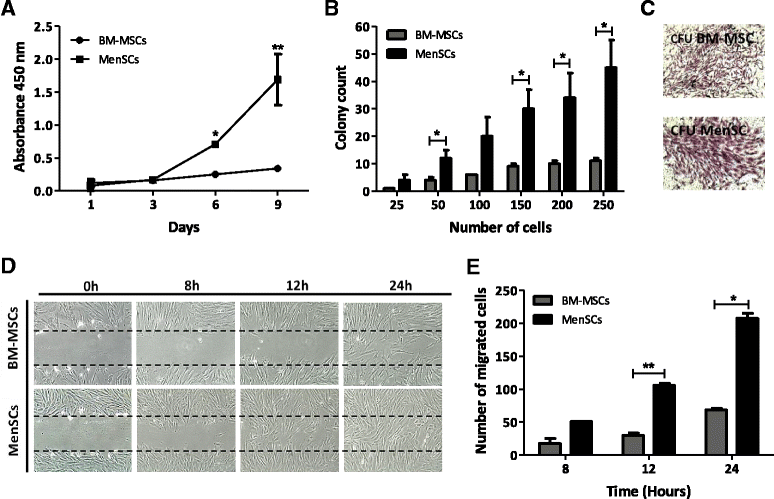
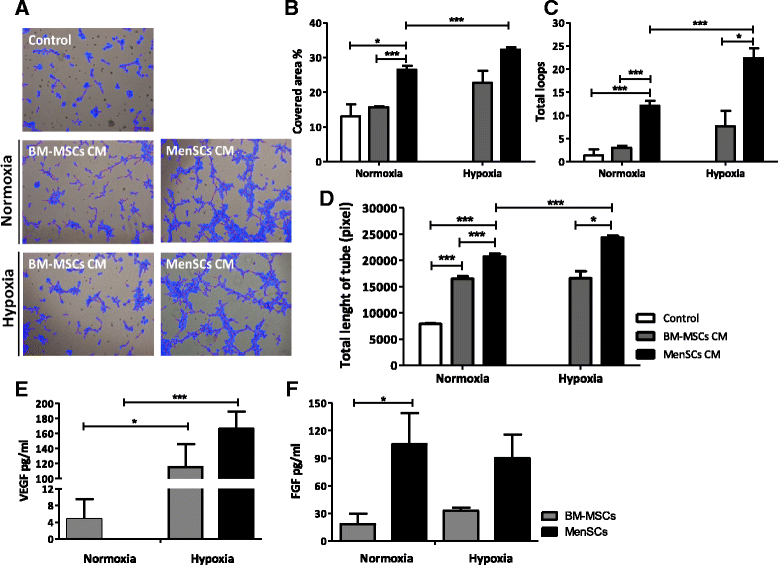
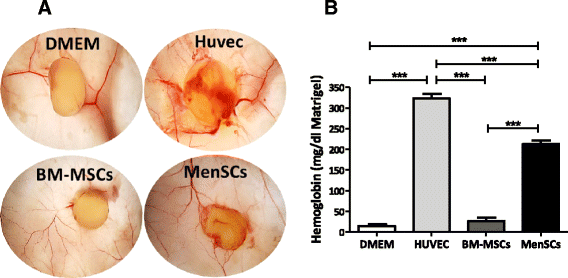
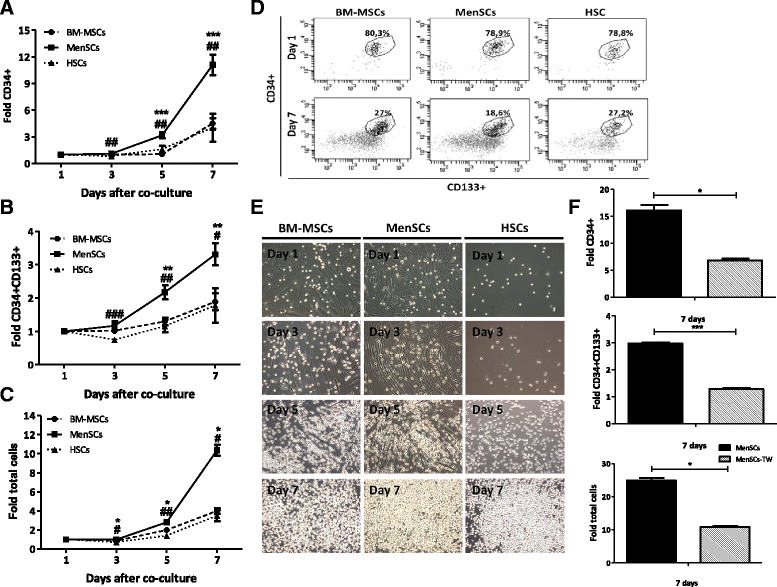
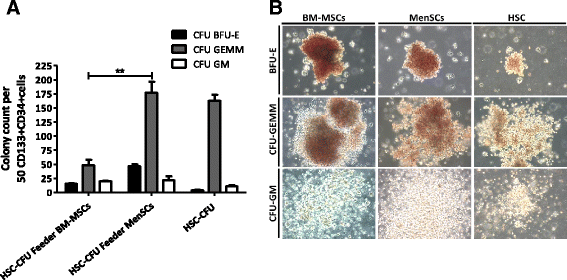
Results: The phenotypic analysis of MenSCs revealed a profile largely similar to the BM-MSCs with the exception of a higher expression of the adhesion molecule CD49a (alpha1-integrin). Furthermore, the fibroblast colony forming units (CFU-F) from MenSCs yielded a 2 to 4 fold higher frequency of progenitors and their in vitro migration capacity was superior to BM-MSCs. In addition, MenSCs evidenced a superior paracrine response to hypoxic conditions as evidenced by the secretion of vascular endothelial growth factor and basic fibroblast growth factor and also improved angiogenic effect of conditioned media on endothelial cells. Furthermore, MenSCs were able to induce angiogenesis in a matrigel plug assay in vivo. Thus, an 8-fold increase in hemoglobin content was observed in implanted plugs containing MenSCs compared to BM-MSCs. Finally, we demonstrated, for the first time, the capacity of MenSCs to support the ex-vivo expansion of HSCs, since higher expansion rates of the CD34+CD133+ population as well as higher numbers of early progenitor (CFU-GEMM) colonies were observed in comparison to the BM source.
Conclusions: We present evidence showing superiority of MenSCs with respect to several functional aspects, in comparison with BM-MSCs. However, the impact of such properties in their use as adult-derived stem cells for regenerative3 medicine remains to be clarified.
Figures
References
-
- Akimoto K, Kimura K, Nagano M, Takano S, To’a Salazar G, Yamashita T, et al. Umbilical cord blood-derived mesenchymal stem cells inhibit, but adipose tissue-derived mesenchymal stem cells promote, glioblastoma multiforme proliferation. Stem Cells Dev. 2013;22:1370–86. doi: 10.1089/scd.2012.0486. - DOI - PMC - PubMed
-
- Cui CH, Uyama T, Miyado K, Terai M, Kyo S, Kiyono T, et al. Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol Biol Cell. 2007;18:1586–94. doi: 10.1091/mbc.E06-09-0872. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

