Cultivation strategies for production of (R)-3-hydroxybutyric acid from simultaneous consumption of glucose, xylose and arabinose by Escherichia coli
- PMID: 25889969
- PMCID: PMC4405896
- DOI: 10.1186/s12934-015-0236-2
Cultivation strategies for production of (R)-3-hydroxybutyric acid from simultaneous consumption of glucose, xylose and arabinose by Escherichia coli
Abstract
Background: Lignocellulosic waste is a desirable biomass for use in second generation biorefineries. Up to 40% of its sugar content consist of pentoses, which organisms either take up sequentially after glucose depletion, or not at all. A previously described Escherichia coli strain, PPA652ara, capable of simultaneous consumption of glucose, xylose and arabinose was in the present work utilized for production of (R)-3-hydroxybutyric acid (3HB) from a mixture of glucose, xylose and arabinose.
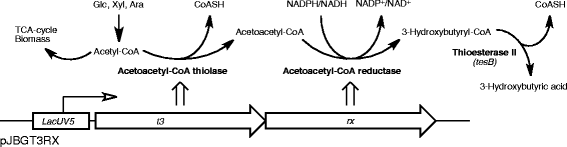
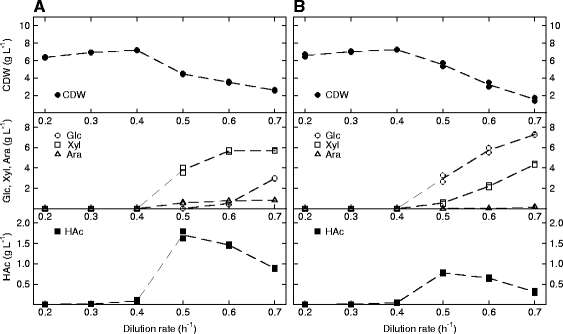
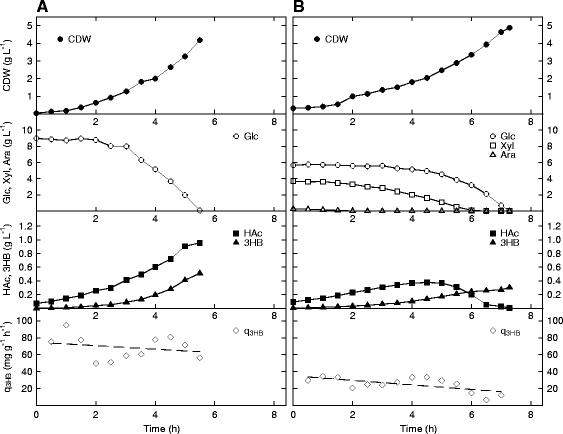
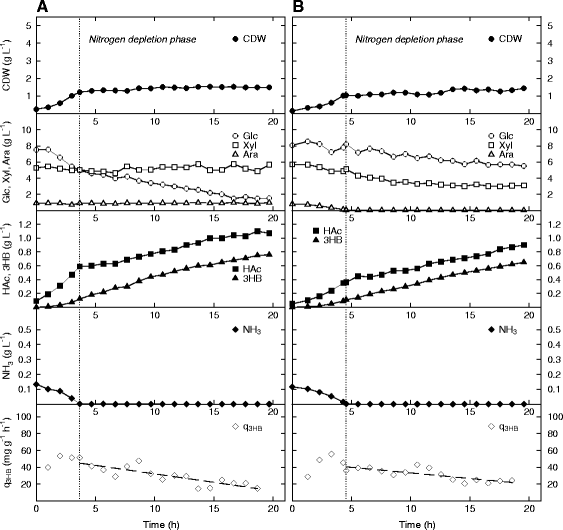
Results: The Halomonas boliviensis genes for 3HB production were for the first time cloned into E. coli PPA652ara, leading to product secretion directly into the medium. Process design was based on comparisons of batch, fed-batch and continuous cultivation, where both excess and limitation of the carbon mixture was studied. Carbon limitation resulted in low specific productivity of 3HB (<2 mg g(-1) h(-1)) compared to carbon excess (25 mg g(-1) h(-1)), but the yield of 3HB/cell dry weight (Y3HB/CDW) was very low (0.06 g g(-1)) during excess. Nitrogen-exhausted conditions could be used to sustain a high specific productivity (31 mg g(-1) h(-1)) and to increase the yield of 3HB/cell dry weight to 1.38 g g(-1). Nitrogen-limited fed-batch process design led to further increased specific productivity (38 mg g(-1) h(-1)) but also to additional cell growth (Y3HB/CDW=0.16 g g(-1)). Strain PPA652ara did under all processing conditions simultaneously consume glucose, xylose and arabinose, which was not the case for a reference wild type E. coli, which also gave a higher carbon flux to acetic acid.
Conclusions: It was demonstrated that by using E. coli PPA652ara, it was possible to design a production process for 3HB from a mixture of glucose, xylose and arabinose where all sugars were consumed. An industrial 3HB production process is proposed to be divided into a growth and a production phase, and nitrogen depletion/limitation is a potential strategy to maximize the yield of 3HB/CDW in the latter. The specific productivity of 3HB reported here from glucose, xylose and arabinose by E. coli is further comparable to the current state of the art for production from glucose sources.
Figures


Similar articles
-
Increasing the production of (R)-3-hydroxybutyrate in recombinant Escherichia coli by improved cofactor supply.Microb Cell Fact. 2016 Jun 1;15:91. doi: 10.1186/s12934-016-0490-y. Microb Cell Fact. 2016. PMID: 27245326 Free PMC article.
-
Simultaneous uptake of lignocellulose-based monosaccharides by Escherichia coli.Biotechnol Bioeng. 2014 Jun;111(6):1108-15. doi: 10.1002/bit.25182. Epub 2014 Jan 23. Biotechnol Bioeng. 2014. PMID: 24382675
-
Recombinant Ralstonia eutropha engineered to utilize xylose and its use for the production of poly(3-hydroxybutyrate) from sunflower stalk hydrolysate solution.Microb Cell Fact. 2016 Jun 3;15:95. doi: 10.1186/s12934-016-0495-6. Microb Cell Fact. 2016. PMID: 27260327 Free PMC article.
-
Ethanol production from lignocellulosic biomass by recombinant Escherichia coli strain FBR5.Bioengineered. 2012 Jul-Aug;3(4):197-202. doi: 10.4161/bioe.19874. Epub 2012 Jun 18. Bioengineered. 2012. PMID: 22705843 Free PMC article. Review.
-
Arabinose as an overlooked sugar for microbial bioproduction of chemical building blocks.Crit Rev Biotechnol. 2024 Sep;44(6):1103-1120. doi: 10.1080/07388551.2023.2270702. Epub 2023 Nov 6. Crit Rev Biotechnol. 2024. PMID: 37932016 Review.
Cited by
-
Engineering Escherichia coli for production of 4-hydroxymandelic acid using glucose-xylose mixture.Microb Cell Fact. 2016 May 27;15:90. doi: 10.1186/s12934-016-0489-4. Microb Cell Fact. 2016. PMID: 27234226 Free PMC article.
-
Regulating the production of (R)-3-hydroxybutyrate in Escherichia coli by N or P limitation.Front Microbiol. 2015 Aug 19;6:844. doi: 10.3389/fmicb.2015.00844. eCollection 2015. Front Microbiol. 2015. PMID: 26347729 Free PMC article.
-
Comparison of engineered Escherichia coli AF1000 and BL21 strains for (R)-3-hydroxybutyrate production in fed-batch cultivation.Appl Microbiol Biotechnol. 2019 Jul;103(14):5627-5639. doi: 10.1007/s00253-019-09876-y. Epub 2019 May 18. Appl Microbiol Biotechnol. 2019. PMID: 31104101 Free PMC article.
-
Active pharmaceutical ingredient (API) chemicals: a critical review of current biotechnological approaches.Bioengineered. 2022 Feb;13(2):4309-4327. doi: 10.1080/21655979.2022.2031412. Bioengineered. 2022. PMID: 35135435 Free PMC article. Review.
-
The role of the acyl-CoA thioesterase "YciA" in the production of (R)-3-hydroxybutyrate by recombinant Escherichia coli.Appl Microbiol Biotechnol. 2019 May;103(9):3693-3704. doi: 10.1007/s00253-019-09707-0. Epub 2019 Mar 5. Appl Microbiol Biotechnol. 2019. PMID: 30834961 Free PMC article.
References
-
- Garcia Sanchez R, Karhumaa K, Fonseca C, Sànchez Nogué V, Almeida JR, Larsson CU, et al. Improved xylose and arabinose utilization by an industrial recombinant Saccharomyces cerevisiae strain using evolutionary engineering. Biotechnol Biofuels. 2010;3:13. doi: 10.1186/1754-6834-3-13. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

