Clozapine toxicity due to a multiple drug interaction: a case report
- PMID: 25890012
- PMCID: PMC4393570
- DOI: 10.1186/s13256-015-0547-2
Clozapine toxicity due to a multiple drug interaction: a case report
Abstract
Introduction: We report the case of a multiple drug interaction involving clozapine, antifungals and oral contraceptives, which resulted in an increased clozapine plasma level, pericarditis with pericardial effusion and eosinophilia in a young Caucasian woman. These symptoms and signs disappeared a few days after discontinuation of clozapine. At present, we are not aware of reports of clozapine-antifungals interaction, whereas there is only one other case report on the interaction between oral contraceptives and clozapine. The purpose of this case report is to show the risk of potentially serious adverse effects stemming from drug interactions involving medications routinely used in clinical practice.
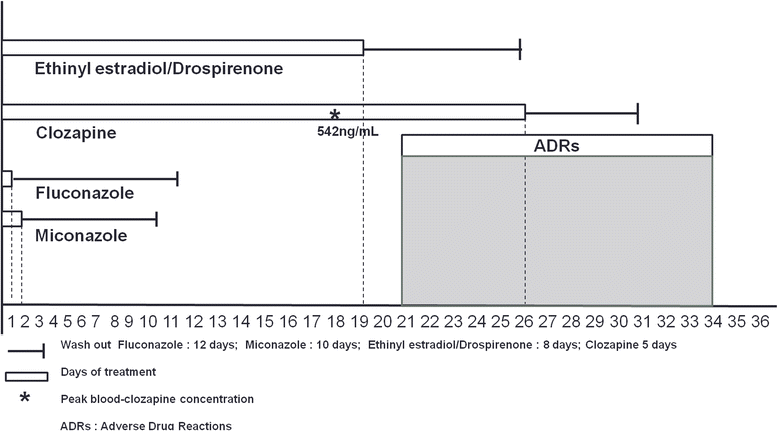
Case presentation: A 29-year-old Caucasian woman diagnosed with a schizoaffective disorder was admitted to a psychiatric unit for acute psychosis (hallucinations, delusions and catatonic behavior). She denied smoking tobacco products and was on long-term oral contraceptives. During the first month of hospitalization she was treated with antipsychotics and for 1 week she took simultaneously fluconazole and miconazole gel, after being diagnosed with oral candidiasis. On the last day of antifungals treatment, 29 days after admission, clozapine was started with resolution of psychotic symptoms. After 3 weeks, her clozapine plasma level had increased to 542 ng/mL and eosinophilia was observed. She complained of nausea, vomiting and palpitations; echocardiography showed echocardiographic abnormalities and pericardial effusion. Oral contraceptives were discontinued and after 1 week clozapine was interrupted, with a complete resolution of side effects and pericardial effusion within 4 days.
Conclusions: Clozapine is metabolized by cytochrome P450. The use of inhibitors or other substrates of cytochrome P450, such as antifungals and oral contraceptives, can cause long-lasting interactions and clozapine toxicity. The Naranjo algorithm shows clozapine is a definite cause of pericarditis (score 9) and both clozapine-antifungals and clozapine-contraceptives interactions resulted probable (score 5) in Drug Interaction Probability Scale. A good knowledge on drugs that act as substrates, inhibitors or inducers of cytochrome P450 is mandatory. When those drugs are used in patients taking clozapine, blood level monitoring of clozapine should be recommended, since a lower dose of clozapine might be required to prevent clozapine toxicity.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

